Introduction
Acute promyelocytic leukaemia, a subtype of acute myelocytic leukaemia (AML) that usually occurs in patients younger than 40 years of age, accounts for approximately 10-15% of AML cases. Acute promyelocytic leukemia is characterised by the chromosomal translocation t(15;17). The translocation occurs between the retinoic acid receptor a (RARA) gene on chromosome 17 and the promyelocytic leukaemia (PML) gene on chromosome 151-3. This rearrangement results in a leukaemogenic PML-RARA fusion gene which alters the growth and differentiation of certain myeloid cells3. The hallmark of APL is the uncontrolled proliferation of abnormal promyelocytes and disseminated intravascular coagulopathy (DIC)1,4,5. Prior to the introduction of ATRA therapy, APL was the most aggressive form of AML with death resulting from severe coagulopathy and bleeding diathesis on average in one month1. Effective treatment for APL with ATRA was developed between the years 1983-19881,6. Until recently therapy included ATRA plus chemotherapy as standard. However, recent trials support the non- chemotherapeutic ATRA + arsenic trioxide (ATO) combination7. Patients are stratified into low/ intermediate risk, or high risk with a white cell count (WCC) less than 10,000 per microliter (mL)., or greater than 10,000 per microliter (mL). respectively.
Patients with a high WCC are particularly at risk of fatal early haemorrhagic complications, and this risk is reduced with early ATRA therapy. Thus, rapid diagnosis and commencement of ATRA is vital in this disease to reduce the early mortality that used to occur. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukaemia7-10.
The authors have looked to the aviation community for strategies to improve patient care in complex situations. Crew resource management (CRM) is a fundamental set of training procedures used in the aviation environment which aims to optimise the effectiveness of coordinated efforts to obtain shared goals. This case highlights the importance of team work and collaboration to ensure effective treatment and a good outcome when time is a critical factor. Because military health care infrastructure in the United States is complex and relies heavily on civilian medical services early implementation of military medical resource management (MMRM) enhances patient care especially in cases that require rapid diagnosis and treatment such as APL.
Case Report
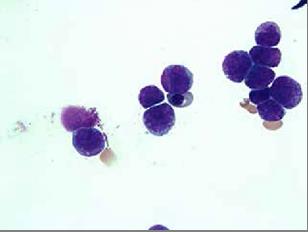
The patient, a 38 year-old Air Force active duty male, was in his usual excellent state of health until the end of April 2013 when he presented to the flight medicine clinic located in the continental United States with shortness of breath, fatigue, unexplained bruising of the legs, and mild bleeding of the gums. At that time complete blood count (CBC) revealed pancytopenia with a predominance of promyelocytes on peripheral smear which was indicative of leukaemia. White cell count 2.32 x 103 / uL with 48% promyelocytes, Hb8.6 g/dL, and platelets 38×103/uL. During the repeat blood draw in the clinic lab, the patient began to feel weak and was taken to the emergency department via ambulance. In the emergency department the patient experienced an episode of haematochezia and the decision was made to transfer the patient to a nearby hospital with in-patient haematology services. The following morning, a bone marrow biopsy (Figures 1&2) was obtained and fluorescence in situ hybridization (FISH) using gene specific probe analysis confirmed the diagnosis of APL. The patient was promptly started on induction therapy with ATRA at 45 mg/ m2/day plus arsenic at 0.15 mg/kg/day. Additional laboratory analysis was significant for early DIC which normalised within a week of therapy. Induction therapy was complicated by a minimally symptomatic transaminitis which required a 3 day break in therapy; complete remission was confirmed by bone marrow biopsy which showed complete cytologic remission at day 35 of induction therapy. Two weeks following the completion of induction therapy the patient started consolidation therapy which consisted of a continuation of arsenic trioxide (0.15 mg/kg/day IV daily for 5 days per week for 4 weeks every 8 weeks) for a total of 4 cycles and ATRA (45 mg/m2/day for 2 weeks every 4 weeks) for a total of 7 cycles. The patient tolerated the treatment extremely well and his prognosis is considered to be excellent.
Discussion
Acute promyelocytic leukaemia is a potentially fatal disease which requires prompt diagnosis and treatment at a facility that supports inpatient haematology services. Flight medicine cases such as this require aggressive resource management and coordination of care. Crew resource management (CRM) is an aviation term which relates to communication and efficient utilisation of flight equipment and crew11. Because of its effectiveness, CRM has been widely adopted across civilian and military aviation. It can be defined as a system which makes optimal use of all available resources, equipment, procedures, and people. Effective resource management promotes safety and enhances the efficiency of operations. Because the primary aim of military medicine is to maintain the health of military personnel so that they can carry out their mission, proper military medical resource management should be implemented especially in deployed locations or resource- depleted regions of the world.
The focus of MMRM is to ensure clear, efficient, and timely communication across disciplines which can be applied to flight medicine. While not every flight medicine case will require intense MMRM, APL is a good example of one that does. In this case, the primary flight medicine team served as the gatekeeper to the patient’s access to timely diagnosis and definitive treatment. Prompt communication with the lab, emergency department physician, haematologist, insurance representatives, and the patient’s commander were essential to effective management of the situation. Time was a significant factor and hours did matter. A barrier to the proper implementation of MMRM is delayed identification of potentially fatal non-traumatic disease pathologies in an extremely healthy operator population. Communication with the clinic laboratory director about the initial peripheral smear allowed immediate engagement of a haematologist and activation of the referral management team. Keeping the referral team up to date facilitated insurance authorisations ensuring uninterrupted patient care. Continued communication with referral management, the haematologist, and the patient’s military commander were important throughout the duration of treatment. It is the author’s opinion that regular implementation of MMRM will promote flight medicine’s objective: to support the mission.
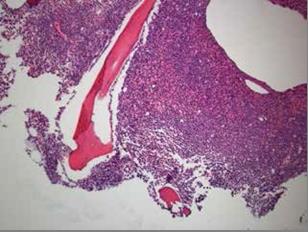
Figure 1: Low power slide which demonstrates hypercellular bone marrow comprised predominately of atypical promyelocytes.