INTRODUCTION
JAPANESE ENCEPHALITIS QE) virus causes an endemic and epidemic zoonotic disease with a simple life cycle. The typical reservoir is pigs, though horses and dogs were also found to have seroconverted to the virus in northern Australia and Papua New Guinea where the disease has probably emerged from being epizootic to enzooticu. Adreit birds are an intermediate host and transmit the virus over long distances with their migratory habits perhaps explaining the introduction into the Australian region.3 Several Marsupial species have been tested for seroconversion to JE with possums found to have responded in the north of Australia. Transmission between mammals (and possibly marsupials) and humans is by culicine mosquitoes, specifically, the virus was isolated from Culex annulirostris in the Torres Strait in 1995″‘.
CLINICAL FEATURES
Japanese encephalitis is a febrile illness associated with central nervous system irritation. The incubation period varies from five to fifteen days.5.6 Typically, the clinical syndrome begins with a mild febrile illness with headache, rhinorrhoea and cough consistent with a mild respiratory illness. Occasionally, the syndrome will include vomiting and diarrhoea. These symptoms may then be overwhelmed by neurological manifestations from meningeal irritation, meningism, and various generalised and focal encephalitis symptoms such as confusion and coma, and paralyses, Parkinsonian dyskinesia and seizures.7 The syndrome may present as an acute flaccid paralysis and be misdiagnosed as poliomyelitis”.
Children are the highest incident group suffering Japanese encephalitis in endemic areas, though the disease is not truly a childhood disease as non-immune adults entering endemic or epidemic areas are also at risk. Thus, vaccination targets early in the paediatric schedule and for travellers, military personnel and expatriates exposed to JE endemic areas. A third group could be added to receive vaccination programs, those adults and children of areas into which JE expands, such as on the sub-continent of Asia.
An infection may vary from being sub-clinical to causing death from encephalitis in ratios between 1:25 among naive US military personnel serving in Korea11 and 1:100010, though it is commonly quoted at around 1:3009.
Both increased surveillance for acute neurological syndromes searching for polio and the subsequent control of this virus have increased awareness of Japanese encephalitis and it is now the commonest cause for encephalitis in Asia.
EPIDEMIOLOGY OF JAPANESE ENCEPHALITIS
Japanese encephalitis was first recognized in 1935 in Japan and has been recognised as an emerging infection in the region. Slim and Solomon categorised two epidemiological patterns of JE, a northern temperate zone form of large epidemics in summer and a southern hemisphere tropical pattern of endemicity with rainy season peaks. The northern countries with the temperate pattern include Japan, Taiwan, China, Korea, northern Vietnam, northern Thailand, Nepal and northern India. The southern tropical countries with tropical endemic patterns are southern Vietnam, southern Thailand, Indonesia, Malaysia, Philippines, Sri Lanka and southern India. The CDC map (Figure 1) demonstrates the overall distribution well. The categorisation fails to recognise that the while the advancing southern margins of JE virus distribution has not yet reached the Tropic of Capricorn, the epidemiology in the Australasian region more closely reflects that which was described as a “northern temperate” pattern.
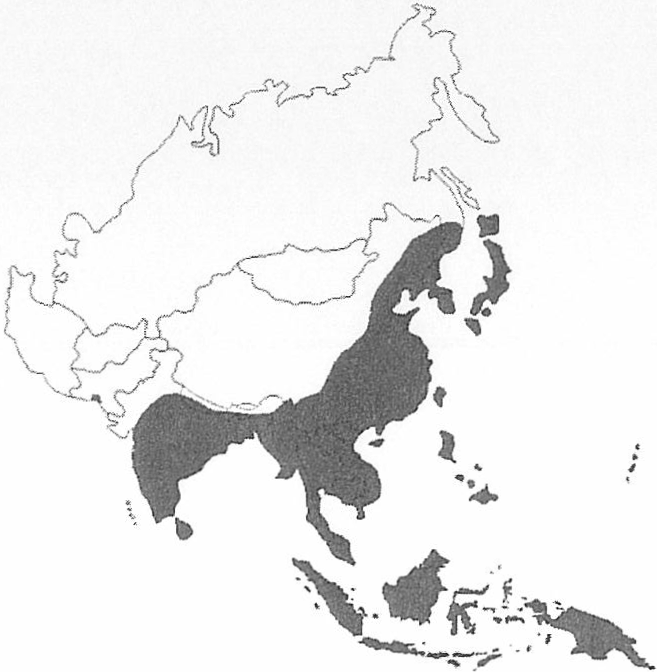
Figure 1: Distribution of Japanese Encephalitis in Asia, 1970-1998
CONTEMPORARY MILITARY SIGNIFICANCE
Mosquito control provides a degree of protection against personnel losses due to JE; however, this is not readily available over the large areas required for efficacy. Similarly, vaccination of pig populations is not a ready option for deploying forces. Consequently, the Australian Defence Force embarked on large-scale vaccination of deploying forces to endemic areas of Southeast Asia16 Australian Defence Service personnel are vaccinated according to the National Health and Medical Research Council Guidelines – an initial course of vaccination is three 1.0ml subcutaneous injections on days 0, 7 and 28.17 There is only one licenced JE vaccine in Australia (JE-VAX”‘), the cost of which represented 42% of the budget for vaccines in the ADF in FY98/99′”. As JE vaccination expends a major portion of the budget for vaccines available to the ADF Health Service, the disease must be considered one of military significance; however, this is not the only impact of JE upon the military determining significance.
HISTORIC MILITARY SIGNIFICANCE
Okinawa
In the final months of war in the Pacific, Allied Forces, predominantly American troops, invaded the small tropical island of Okinawa. Japanese public health physicians were aware of epidemic “Encephalitis Lethargica” in the summer months in the island group of Okinawa so that the development of cases on two of these islands (Heanza and Hamahika) in July was not unusual. The first non-battle casualty of the American Invasion Force in Okinawa died from Japanese encephalitis on 7 August 1945.9 The JE casualty list provides some insight into the nature of an outbreak among non-immune military personnel with 38 suspected of developing a viral infection of the nervous system, 12 with moderate to severe encephalitis and two died. Nine surviving cases and both of those succumbing to the encephalitis were shown to have serological evidence of infection with Japanese B encephalitis virus.
Major Albert Sabin had previously been involved in developing a vaccine against “the Japanese B type of Epidemic Encephalitis” and finally prepared a liquid vaccine suspending 10% mouse brain containing the virus inactivated by formaldehyde. With the circumstances rapidly evolving on Okinawa, an uncontrolled administration of the vaccine was staged with fewer than 1000 “military government” personnel in late July 1945, before two 2cc doses of the vaccine were given to between 60000 and 70000 persons. From the reports of 53139 persons receiving two doses and 2274 receiving one dose, 61 local reactions of mostly pain and five cases of secondary infection were gathered. Sabin noted that reporting might have been variable as an Army Division reported far fewer reactions than a Marine Division. He concluded that as mouse brain solutions had not been previously used, the 19 allergic reactions were more likely due to the formaldehyde commonly used for inactivating other vaccines for US Forces. As the last military case occurred on August 21 and the general vaccination program did not start until the second week of August, the epidemic was probably not controlled by the vaccine but rather by natural diminution.
US FORCES IN KOREAc
In the following summer, another 250000 persons were vaccinated in Japan and health intelligence of the distribution of JE was gathered in the form of serological evidence around the Asian region. However, no evidence was found of the virus on the Korean Peninsula, so that when the American Forces deployed in the next year, they were not vaccinated against JE21. Late in August of 1946, four cases of encephalitis occurred among those deployed. Three of these cases had developed within one week of each other in an isolated facility near Kunsan on the Yellow Sea coast. Rains had been heavy in the preceding June. A subsequent cholera outbreak in the city kept personnel on the base, as did impassable roads. July and early August were hot and dry, though the rice paddies close to the camp remained soaked. The radius of DDT spraying around the camp was inadequate to prevent mosquitoes infesting the Lines on the evening breezes. Accommodation huts, an outdoor theatre and the service club were either unscreened or inadequately so. When Sabin arrived at the camp shortly after the cases presented, he and the entomologist found Culex tritaeniorhynchus larvae in the area. Based on clinical and epidemiological findings, without serological support or isolation of virus, a vaccination program was started before the end of August using the inactivated mouse-brain vaccination derived from the Nakayama strain previously isolated, successfully transfected to the mouse model and scaled up moderately for production quantities of vaccine. A two-dose schedule with three to five days’ separation was employed and no further cases of encephalitis occurred, though three cases among 1500 soldiers may have been the limit of the outbreak naturally. With the closeness of presentation of cases, vaccination was concluded to be useful in prevention rather than outbreak control – as with the previous outbreak in Okinawa.
The US Forces apparently did not continue vaccination against J E as in 1958 Halstead retraced Sabin’s footsteps in travelling to Kunsan Air Base to investigate three cases of JE among the 800 personnel on base during August of that year. In a sample of 300 personnel present on the base throughout the transmission period July to September inclusive), 28 were found to have antibodies by haemagglutination inhibition testing. None of the people had been previously posted to JE endemic areas. With three overt infections and an estimated 75 sub-clinical infections, the clinical: sub-clinical ratio for Kunsan in 1958 was 1:25. Airmen found to have been infected were significantly more likely to have experienced a systemic illness during the transmission period compared to those without antibodies. In particular, respiratory symptoms were common with patients presenting with mild illnesses in this period and later found to have antibodies to JE. Occupations requiring duties outdoors were four times more likely to have antibodies. Army Engineers were especially overrepresented in this group.
AUSTRALIANS IN VIETNAM
Japanese encephalitis was recognised as a health risk of operations in Vietnam including on redeployment’s. Fifty-seven cases were recorded in 1969 from approximately 10000 deployed troops with varying individual exposures. The delay in laboratory diagnosis for JE caused problems for clinicians dealing with pyrexia of unknown origin25 providing only retrospective diagnosis for clinical review26 and contributing to operational epidemiology. Specific operational epidemiology was determined during the deployment of Sixth Battalion, Royal Australian Regiment (6RAR) into Phuoc Tuy Province of Vietnam from April 1966″. The Battalion underwent a “febrile illness study” entailing blood samples taken at the conclusion of the deployment to determine infectious disease exposures. During this period, no cases of JE were reported; however, 20.9% of the soldiers had been infected. Over the Task Force (n-2000 personnel) in the province including the Vung Tau base, an estimated 420 infections would have occurred. Extrapolating this information to the clinical ratio for Australian soldiers deployed in Vietnam in 1966, clinical infection serious enough to be directly identified as JE is less common than 420:1.
Ironically, a case of Japanese encephalitis was described in a soldier returned from Vietnam with the conclusion that even though the disease represents a serious hazard in deploying non-immune soldiers, it would be unlikely to become a problem in Australia.
THE BRITISH EXPERIENCE IN NEPAL
Health intelligence from domestic epidemiology was generated by the Royal Army Medical Corps in Nepal to identify intense transmission in the area of Dharan. While most cases were children, active immunisation was recommended for the non-immune soldiers and families. Henderson then went on to vaccinate 1152 people with lyophilised Biken JE vaccine, resulting in 90% of recipients being immune after three vaccinations, yet 30% of the Nepalese were already immune. Perhaps this pre-immune rate inflated the success rate.
US FORCES IN SE ASIA AND THE WESTERN PACIFICc
After the British experience in Nepal, the findings of the clinical efficacy field study conducted by Hoke and colleagues in Thailand were available; however, the US military recognised that this represented the response among flavivirus primed exposed individuals. To provide more applicable data, 4034 US soldiers were vaccinated with two doses of the mouse brain inactivated Nakayama strain vaccine one week apart. The study was conducted over two years from 1987. Sixteen of 20 soldiers had titres reflecting immunity seven weeks after the two doses of vaccine and only nine of 27 retained immunity by six months. At this time, a booster was given which slowly achieved full immunisation of all tested (25) by four weeks. Likely to meet an operational conclusion, two doses were determined to provide adequate immunity “between eight and 12 weeks after (onset of immunization). The cost of the vaccine and duration of this full course (six months) would make the three-dose schedule not operationally viable.
The US military have maintained a presence on Okinawa since the invasion in 1945. In 1991, three cases of JE were sustained among the 20000 Marines on Okinawa.33Again, an emergency immunization campaign was conducted, though on a voluntary basis with an associated prospective study of adverse events. While only 14249 Marines chose to receive JE vaccine, no further cases presented. Notably, 38 recipients had reactions including 26 urticaria and 11 pruritis, which totalled ten times the number of cases prompting the program. During this period, three severe hypersensitivity reactions were recorded raising concern with the vaccination program considering the incidence of clinical infection.
SHORT COURSE VACCINATION
Operational imperatives have entered the research priorities of the US military. The risks and financial cost of widespread vaccination preclude a policy of Army-wide JE immunisation. Defraites and colleagues in the US military explored more operationally suitable pre-exposure short courses35. A 14-day, three-dose course (1.0ml on days 0, 7 and 14) was found to produce a lower geometric mean titre, though an equivalent number of immunes to the longer course over one month.
BOOSTING
On an opportunity basis, a small cohort of the original US soldiers vaccinated to determine an appropriate schedule were reviewed when they had contributed sera to an HIV studio. Only 17 soldiers were directly contacted for review and several confounders such as accurate flavivirus and flavi-vaccine exposure were not controlled. The conclusion was that, as 16 retained immunity three years after vaccination, this was a suitable time for boosting and all were offered another vaccination. Through the NH&MRC Guidelines for immunisation, this study forms the policy for boosting within the ADE
THE FUTURE IN VACCINES
While there are high stakes in human suffering from J E in Asia, these, unfortunately, are not matched by financial rewards for vaccine development. The costs of vaccination and the associated restrictions to duty and adverse event profile have been addressed by the Army Malaria Institute with a series of research studies investigating intradermal vaccination for initial and boosting vaccination37. The findings are promising though require and are undergoing scientific peer review before consideration for policy. The AMI is currently conducting phase 2 trials of a chimeric vaccine constructed from yellow fever vaccine replicating JE envelope proteins. Liveattenuated JE vaccines (SA14-l4-2 strain) are used in China; however, these are not readily scaled for manufacture and would not be suitable for licensure in Australia. Inactivated versions of this vaccine have completed phase 2 trials for registration in the US. Several programs re-adapting the inactivated mouse brain vaccine, available in Australia, to up-scaled growth in non-mammalian industrial cell lines have been successfully moved into Phase 3 in Japan, though the same schedule of vaccination will be retained.
CONCLUSIONS
The case for Japanese encephalitis as a difficult disease of military significance is not persuasive as the incidence in military populations is very low and the operational impact of non-battle casualties negligible to Australian expeditionary forces. However, the vaccination cost is relatively high, consuming a significant proportion of the vaccine budget. Northern Australia is an emergent endemic region for JE so that many Australians are now aware of the disease. Even though currently available vaccination probably does not prevent the viremic state, the importation of viremic Australian soldiers to continental Australia may be publicly unacceptable and a case of Japanese encephalitis in an Australian soldier would probably attract a high profile. For the continued protection of Australian Defence personnel deploying to JE endemic and potentially endemic areas, the Defence Health Service continues to vaccinate and recommend other vector control and personal protection measures against this disease of military significance.






