R Esmaeelzadeh, K Azizbeigi, S Atashak, F Dehghan, F Feizolahi, M A Azarbayjani, Z Khojasteh
Abstract
The present study aimed to investigate the short-term influence of garlic supplementation on oxidative stress markers following military physical activity among military women. Twenty women were randomly assigned to two groups of (1) placebo (control), and (2) garlic extract supplement (experimental) along with a physical activity program. The women in the experimental group took garlic extract supplement daily for 14 days. The control group received a placebo containing 500 mg dextrose in a randomised double-blind placebo-controlled design. Blood samples were collected and analysed for plasma Malondialdehyde (MDA) and 8-hydroxy-2- deoxyguanosine (8-OHdG) before the program and 14 days after.
The results showed that military physical activity caused a significant increase in 8-OHdG and MDA (p=0.001). Hence a significant difference was found between the placebo and supplement groups in MDA (p=0.001) and 8-OHdG (p=0.001) after exercise.
It was concluded that although garlic extract could not prevent oxidative stress, it could attenuate the detrimental effects of oxidative stress among military people during physical activity.
Keywords: Antioxidant, Malondialdehyde (MDA), 8-hydroxy-2’-Deoxyguanosine (8-OHdG), Military training, Garlic
Introduction
It is almost axiomatic that physical fitness is the base of all military education. A low level of physical fitness results in unsuccessful performance in the field by military people. In contrast, good physical fitness can improve soldiers psychic resistibility for fulfilling tasks and combat actions.1 Military programmers have designed various ways to sustain the physical fitness of military personnel. One of these is long-term military physical activity training. However, it has been reported that physical activity and exercise training, especially high-intensity and long-term, may produce free radicals and reactive oxygen species (ROS), and induce oxidative stress in the active muscles and circulation.2 The oxidative activity of ROS is a part of the natural function of cells, but if it is repetitive, ROS can damage other cells’ natural function.3 In fact, the oxygen consumption level and metabolic rate may considerably increase during exercise.4 This may lead to increased superoxide anion production in mitochondria and increase oxidative stress.5 The body’s antioxidant capacity is overwhelmed by the ROS enhancement in this situation. ROS contribute to fatigue because loss of function can be delayed by ROS-specific antioxidants,6 and reduce body function. It is also thought to play an essential role in the pathophysiology of muscle damage and fatigue during activity.7 Studies have reported that high- intensity exercise like military training pushes the lactate level beyond its threshold, leading to more oxidative stress than that caused by moderate aerobic exercise.8 Also, it has been reported that sustained training load during the last four weeks of basic military training leads to oxidative stress observable both at rest and after submaximal exercise.9 Some studies have reported that there is unique oxidative stress in military personnel (e.g. severe air pollution in some urban environments; radiation hazards to crew at altitude; radiofrequency radiation hazards on ships and around communications facilities; lung and tissue blast overpressure effects and physical and psychological stresses in extreme training courses)10 that may have adverse health consequences. Some of these stresses are reasonably well characterised, such as those associated with strenuous exercise, working in the extremes of environmental temperatures, and altitude.10
Biological systems have both enzymatic and non- enzymatic antioxidant defences. In such situations, antioxidants protect against oxidative stress.11 On the other hand, because of the high increase of ROS during physical activity, the antioxidant power is weakened due to oxidative stress. This makes it impossible for the cells to clear the ROS.12 Therefore, researchers have studied a wide range of drug and non-drug antioxidant supplements, which may decrease oxidative stress.13, 14, 15 Some previous studies have reported that the use of a nutrient and antioxidant supplement can be a proper solution to oxidative stress.16 Besides, regular exercise enhances the body’s antioxidant power.17 However, having fewer side effects than synthetic antioxidant components, herbal antioxidants’ use has received attention from several researchers.18 Herbal treatments are getting more attention because of their easy availability, low cost and popularity among consumers.19 Garlic (Allium sativum L.) originated in many regions of the world, especially western Asia and the Mediterranean coast, and has played an important role in ancient and modern medicine.20
Garlic contains different sulfur compounds, such as alliin and allicin, allyl disulfide, terpenes, including citral, geraniol and linalool. Scientists have attributed the antioxidant effects of garlic to thiosulfonate (allicin).21 It has been shown that alliin can scavenge superoxide, while allicin suppresses superoxide formation by the xanthine/xanthine oxidase system. Also, alliin, allyl cysteine and allyl disulfide can all scavenge hydroxyl radicals.22 However, apart from plasma’s antioxidant power, garlic extract consumption may help ameliorate the negative effects of oxidative stress damage after strenuous exercise. This study aimed to investigate the effects of garlic extract consumption on Malondialdehyde (MDA) concentration as a lipid peroxidation, and 8-hydroxy-2’-Deoxyguanosine (8-OHdG) as a DNA damage-marker of military physical activity in military women students.
Methods
Subjects
Twenty female cadet students of the Military Science University voluntarily participated in this study. The subjects were free of drugs and medication, and had no history of endocrine disorders, diabetes, cardiovascular and respiratory diseases, hypertension, and renal and hepatic problems. The subjects had not used any exogenous anabolic- androgenic steroids, antioxidants or dietary supplements and anti-inflammatories, such as NSAIDs and COX-2 inhibitors, six months before the study. The experimental procedures and study protocols, being fully explained to all the subjects, were approved by the Islamic Azad University’s Ethics Committee, Teheran Branch. The subjects had read and understood the details of the experiments before signing a written consent form. The subjects were randomly assigned into two groups of (1) placebo (control), and (2) garlic extract supplement (experimental) along with a physical activity program (n=10 in each group). Since the subjects were living in the dormitory, their diets were controlled. The subjects were asked to decrease their physical activity during supplementation.
Anthropometric measurements
Before the intervention period, all subjects attended the laboratory two days at the baseline. During the familiarisation session, height was measured to the nearest 0.5 cm, without shoes, using a calibrated scale (Cranlea and Company, Bournville, Birmingham, UK). Body mass and total body fat were measured using the X-scan plus II Segmental Body Composition Analyzer (X-scan plus II, Korea), corrected for light indoor clothing. Body mass index (BMI) was calculated as body weight in kilograms divided by height in metres squared (body weight [kg]/height [m]2). VO Cooper VO 2max Test, estimating how far a subject can run/walk in 12 minutes. Based on the distance covered by the subjects, VO2max was calculated by the following formula: VO2max (ml.kg.min.-1) = (Distance covered in metres – 504.9)/44.7323. The subjects’ characteristics and the data obtained from variables based on the mean and the standard deviation (Mean±SD) are presented in Table 1.
Table 1. Characteristics of the subjects before supplementation and physical exercise. Data are expressed
as mean ±SD.
| Group | Age(year) | Weight (kg) | Height (cm) | BMI (kg/m2) | VO2max ml.kg.min-1 |
BF% – |
|---|---|---|---|---|---|---|
| Supplementation | 20.0±1.6 | 55.7±7.1 | 164.3±4.4 | 20.7±2.9 | 47.5±3.5 | 17.5±2.4 |
| Placebo | 20.2±1.5 | 52.6±5.6 | 164.4±4.4 | 19.4±2.1 | 46.7±4.2 | 16.8±3.3 |
| p | – | – | – | 0.30 | 0.42 | 0.65 |
Parade as physical activity
Fourteen days after garlic extract supplementation, the subjects performed a session of physical activity in the form of military parade. The protocol included five-minute warm–up before a 45-minute parade. The subjects were arranged in four columns in a parade line. Each column included five subjects, who started their 60-minute training with movements such as rotation while stopping, rotation while moving, rotation without weapon, shoulder knot, hand knot, shoulder-arm-and-feet knot while stopping and movement with weapon. Once they left the station, they traversed the front of the station in the lockstep. They then completed their parade exercise with movements, such as bending the head, hands and feet at 45-degree angles so the upper and lower extremities contributed continuously to the exercise protocol. The subjects did not have any extra activity during the parade, and they did not drink any liquid. Care was taken to ensure that the environmental conditions of ambient temperature 22–23°C and relative humidity 50–55% were similar during all tests. For this purpose, the parade was conducted indoors so that temperature and humidity control was provided by a sensor (ThermoPro TP50 Digital).
Garlic extract supplement
The supplement group was given one garlic capsule daily—each containing 500 mg of garlic extraction supplement (produced by GOLDARU Company, Iran)—for 14 days. The placebo group received a placebo containing 500 mg of dextrose. The subjects took the capsules with 250 ml water after lunch. The experimental and placebo groups’ capsules were identical in appearance (i.e. size, shape and colour). In preparing capsules, 500 mg of garlic extraction supplement (produced by GOLDARU Company, Iran) was provided. The placebo capsules’ contents, that is, the garlic extract, were emptied and then filled with dextrose. Therefore, the placebo and supplement capsules were somewhat similar in garlic odour. All capsules (both placebo and garlic capsules) were put in the main garlic container and kept near fresh garlic before being delivered to the subjects. Considering that the odour of the placebo capsules might have been reduced after a while, the packages were delivered every seven days for better monitoring and odour control. Thus, garlic odour was one of the limitations of the study, which may have affected the results. The intake timing was standardised to avoid any possible interference with the results, and the dosage was carefully monitored. Each subject received seven capsules at the end of the seven days. The subjects were asked not to have any citrus, caffeine and phenolic compounds during the protocol. Although all subjects were living in the garrison and took a standard diet, to control the amount of the antioxidants consumed, all subjects were asked to complete a validated food intake questionnaire, recording their food intake every day. Diet was analysed using Food Processor software (Esha Research, Salem, USA) with regard to the antioxidant and caloric content. Subjects were also provided with necessary information concerning the amount to be consumed and how to record it during the intervention period to ensure dietary stability. The results of the dietary analysis are presented in Table 2.
Blood sampling and analysis
Blood samples were gathered in three stages: 1) before any intervention (before supplementation and Cooper test) for baseline analysis as pre- test blood samples; 2) 14 days after garlic extract supplementation; and (3) immediately after a session of parade activity. Pre-test blood samples (5 ml) were obtained from the subjects’ antecubital vein in the sitting position before any intervention after a 10- hour fast between 8:00 and 10:00 a.m. Stages 2 and 3 of blood sampling were repeated the same as the pre-test stage by the same technicians.
To measure MDA levels, plasma was separated by centrifugation (1006 gm, 10 min.) immediately after blood sampling at a temperature of 22–23° C and relative humidity of 50–55%. The following procedure measured MDA, according to Yoshioka et al.24 0.5 ml of plasma was shaken with 2.5 ml of 20% trichloroacetic acid (TCA) in a 10 ml centrifuge tube.
Table. 2 Nutrient analysis of the dietary records of the supplement, and placebo groups before and after
supplementation and military exercise period. Data are expressed as mean ±SD.
| Time | supplement | placebo | p | |
|---|---|---|---|---|
| Proteins (g) | Pre supplementation | 112.5±30.5 | 109.8±17.2 | 0.17 |
| After supplementation | 115.0±15.2 | 118.0±18.2 | 0.79 | |
| After exercise | 120.2±18.2 | 118.7±16.5 | 0.44 | |
| Carbohydrates (g) | Pre supplementation | 399.7±55.0 | 410.1±23.8 | 0.06 |
| After supplementation | 402.1±25.1 | 395.5±21.1 | 0.07 | |
| After exercise | 395.1±25.1 | 410.5±11.1 | 0.06 | |
| Fat (g) | Pre supplementation | 120.1±10.3 | 115.1±21.2 | 0.27 |
| After supplementation | 115.2±11.4 | 113.7±7.2 | 0.87 | |
| After exercise | 109.9±10.5 | 111.7±7.2 | 0.11 | |
| Kcal | Pre supplementation | 3129.7±125.5 | 3115.0±85.9 | 0.75 |
| After supplementation | 3105.0±95.5 | 3113.0±107.5 | 0.42 | |
| After exercise | 3050.0±105.8 | 3120.0±115.4 | 0.06 | |
| Vitamin C (mg/d) | Pre supplementation | 60.0±6.8 | 57.0±13.2 | 0.09 |
| After supplementation | 66.5±8.2 | 58.4±10.6 | 0.12 | |
| After exercise | 59.8±7.3 | 61.2±5.9 | 0.33 | |
| α- tocopherol (mg/d) | Pre supplementation | 4.8±1.1 | 4.0±0.8 | 0.77 |
| After supplementation | 5.6±0.9 | 4.4±1.2 | 0.49 | |
| After exercise | 4.4±0.7 | 4.9±0.6 | 0.75 | |
| Vitamin A (µg/d) | Pre supplementation | 492.3±118 | 487.0±143.5 | 0.42 |
| After supplementation | 518.6±132.4 | 475.5±125.4 | 0.06 | |
| After exercise | 499.4±177.8 | 488.4±193.7 | 0.67 |
Comparison of nutrient data between supplement and placebo was done with independent sample t-test. P<0.05.
1 ml of 0.6% 2-thiobarbituric acid (TBA) was added to the mixture, shaken and warmed for 30 min. in a boiling water bath followed by rapid cooling. It was then shaken into a 4 ml nbutyl- alcohol layer in a separation tube. The MDA content in the plasma was determined from the absorbance at 535 nm and 520 nm by a spectrophotometer against butanol. The standards of 5, 10 and 20 nmol/ml tetra ethoxy propane (TEP) were used. The results were expressed as nmol/ml plasma.24 To measure 8-OHdG, plasma was separated by centrifugation at 2000 rpm for 15 minutes. The leukocytes were separated, and their DNA was extracted under the effect of 0.8. Nuclease P1 and phosphatase acid were located in 1 mmol of EDTA and 10 mmol of sodium acetate (pH=4.5) at 37°C for 30 minutes. Then, it was centrifuged at 1500 rpm for five minutes. The supernatant was filtered and infused into the HPLC column. Also, 5 ng/ml of 8-OHdG standard was infused into the HPLC, and the system recorded the peak standard. The results were expressed as ng/ml.
Statistical analysis
Data were expressed as the mean ± standard deviation. First, the normal distribution of all outcomes was confirmed with the Kolmogorov- Smirnov test. The baseline physical characteristics and nutrient data between supplement and placebo groups were compared using an independent sample student’s t-test.
A 2 × 3 (group × time) ANOVA with repeated measures was used to determine the effects of the garlic extract supplement and placebo on the dependent measures. A Bonferroni’s post hoc test was used to identify significant differences when a significant F-ratio was obtained. The statistical significance was set at p≤0.05. All statistical analyses were conducted using the software statistical package SPSS version 19.0.
Results
The dietary intake analysis results showed no significant difference between the placebo and garlic extract supplement groups after supplementation and military physical exercise. There were no differences between the groups at baseline for aerobic power, body weight, body fat percentage, MDA and 8-OHdG (p>0.05).
In addition, there were significant effects of military physical exercise training (p=0.001 and p=0.001, respectively) and interaction of military physical exercise training with supplementation (p=0.001, p=0.001, respectively) on MDA and 8-OHdG levels (Figure 1).

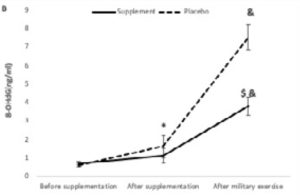
*Denotes significant decrease compared to before supplementation.
& Denotes significant increase compared to after supplementation.
Ṩ Denotes significant decrease compared to the placebo group.
The main effect of time (before and after supplementation and after military exercise) on MDA concentration was statistically significant (F2,36=321.391, p=0.001, PES=0.947. (The main effect of each group (supplementation, placebo) on MDA concentration was statistically significant (F1,18=27.741, p=0.001, PES=0.606). There was a significant interaction between supplement use and time (F2,36=17.890, p=0.001, PES=0.498). The concentration of MDA decreased after taking the supplement and placebo, but the concentration increased significantly after the parade. Although the trend of both groups’ changes was similar, the concentration of MDA in the garlic supplement group after garlic supplementation and after the parade was significantly lower than the placebo group (Figure 1a).
About 8-OHdG, the main effect of time (F2,36=447.847, p=0.001, PES=0.961), group (F1,18=97.003, p=0.001, PES=0.843) and time × group interaction (F2,36=78.931, p=0.001, PES=0.814) was significant. After supplementation or placebo intake, 8-OHdG level increased significantly in both groups, but only after the parade, AAA concentration was significantly lower in the supplement group than in the placebo group (Figure 1b).
Discussion
This study investigated the effects of garlic extract supplementation on oxidative stress markers following military physical activity. The results showed that garlic supplementation could attenuate MDA and 8-OHdG responses after exercise more than placebo, but it could not prevent oxidative stress after activity.
Before any intervention, BMI and body fat mass of the control and experimental groups were homogenous. Although body composition and fat tissue have a direct relationship with oxidative stress levels,25 it appears that BMI and body fat mass did not affect the results. On the other hand, the aerobic power levels of the subjects did not have any significant differences. Thus, the effects of physical fitness and oxygen consumption on the ROS process were controlled.
The results showed that 14 days of garlic extract supplementation could not prevent lipid peroxidation induced by military parade. Compared to the placebo group, the increase in MDA level was significantly lower, and in the absence of garlic extract supplementation, the exercise protocol induced lipid peroxidation and DNA damage. This shows that the intensity and time of activity could be a challenge for the antioxidant system. This study found that exercise induces a measurable increase in the oxidative stress biomarkers, which increased MDA concentration three and a half and five times in response to the supplement and placebo group activity. On the other hand, 8-OHdG concentration showed an increase in the supplement and placebo groups. It was reported that had the intensity of the training been higher, the blood’s oxidative stress indices would have doubled.26 Very high-intensity exercise seems to exaggerate this stress response. Contrary to the present study, Saritaş et al. (2011) reported that MDA concentration significantly decreased after healthy trained men completed a 12-minute Cooper test run27. The inconsistency in the results of the above-mentioned study and the present study is due to the differences in the intensity of exercise or the training status, the participants’ age, BMI and other various indices. It has been reported that all factors, such as the duration and intensity of training, skills, gender, athletic ability and the environmental state, can affect stress-induced oxidative damage.28 Also, oxygen consumption increases during high-intensity aerobic exercise, and consequently, increases the mitochondrial activity in the constricted muscles.2 However, some oxygen consumption during metabolism and the mitochondrial respiratory system in the aerobic organisms may convert into superoxide radicals (O •−), hydrogen peroxide (H O ), hydroxyl radicals (•OH) and singlet oxygen (1O2), and their production may lead to increased oxidative stress.29
The results of this study suggested that garlic extract supplement attenuates levels of MDA oxidative stress and 8-OHdG in comparison with placebo in response to physical activity. The potential mechanism of garlic extract supplementation is that it may reduce the harmful effects of free radicals and oxidative stress related to its antioxidant compounds. This indicates that garlic supplement could enhance the rest level of the antioxidants power or limit the adverse effects of free radicals and ROS production.30 It has been reported that the use of garlic extract can increase some antioxidant enzymes, such as catalase and glutathione peroxidase.31 This can explain why garlic extract supplementation reduced oxidative stress production. On the other hand, it has been reported that some of the compounds in garlic, such as allicin, which are dose-dependent, are most effective in clearing free radicals such as hydroxyl radical.22 It is clear that excess of hydroxyl radical (occurring during exercise) can cause lipid peroxidation, thus damaging cell membranes and lipoproteins.32 Therefore, it seems that allicin could attenuate lipid peroxidation in the supplement subjects by scavenging hydroxyl radical.
In this study, the garlic extract dose was 500 mg a day. Some reports indicated that garlic extract’s effectiveness on the antioxidants indices is sensitive to the dose and the type of antioxidant variable.33 It was suggested that the SOD enzyme activity increases at a low dose.34 However, in this study, we did not measure the antioxidant variables and cortisol level to determine the effectiveness of garlic extract and the relevant mechanisms. This is a limitation and disadvantage of the study. It has been reported that both military women and men are exposed to a wide range of stressor events as a part of military training and work assignments that may affect cortisol secretion.35 Cortisol may increase oxidative damage. On the other hand, it may also initiate up-regulation of antioxidant defences and reduce free radical production. Accordingly, the evaluation of cortisol could further help interpret the results.36 From the related oxidative stress reduction mechanism, it seems, though, that the consumption dose in the present study was successful enough to improve antioxidant status. Nasiri et al. (2017) reported that garlic can reduce oxidative stress and improve total antioxidant capacity, MDA and total oxidant status (TOS) in diabetic rats.37
Lower increase in oxidative stress indices in the supplement group in the present study indicates that garlic supplementation positively affects oxidant/ antioxidant balance. The antioxidant compounds in garlic may have prevented oxidative stress caused by exercise and, consequently, decrease oxidative stress and protect the cell structure. In general, the garlic function mechanism and its declining effects on lipid peroxidation may be due to scavenging of free radicals by sulfur compounds like allicin, S-allyl cysteine and sulfur oil.38 It was reported that garlic and its compounds, including the sulfur factor, help avert lipid peroxidation by inactivating TNF-α and nuclear Factor-Kappa B (NF-kB).39 Therefore, it seems that allicin’s antioxidative mechanism is one of the main features of garlic thiosulfonate that is highly antioxidative, inducing the inhibition of chain–carrying peroxyl radicals and transportation of peroxides. On the other hand, it does not seem that the decrease in lipid peroxidation and 8-OHdG is wholly related to allicin. In this study, these effects may have been the result of the glutathione increment. It has been reported that garlic consumption induces an increase in glutathione, and thus, the body’s antioxidant power increases.39 In general, regardless of garlic compounds, it could be said that garlic supplementation may not exclusively prevent oxidative stress during physical military training. However, as an antioxidant supplement, it could attenuate oxidative stress, accelerate recovery and promote injured tissues’ recovery.40
Conclusion
In spite of some limitations of the present study, such as not checking the plasma total antioxidant capacity, 14 days of consuming garlic extraction could not prevent oxidative stress following long period exercise in the form of military activity. However, garlic supplementation could attenuate lipid peroxidation and DNA damage markers following physical military training.
Corresponding Author: Mohammad Ali Azarbayjani m_azarbayjani@iauctb.ac.ir
Authors: Roghaye Esmaeelzadeh1, Kamal Azizbeigi2, Sirvan Atashak 3, Firouzeh Dehghan4, Foad Feizolahi5, Mohammad Ali Azarbayjani 6, Zohreh Khojasteh7
Author Affiliations:
- Physical education department, Amin Police University, Tehran, Iran
- Exercise physiology Department, Islamic Azad University, Sannandaj Branch, Sannandaj, Iran
- Exercise physiology Department, Islamic Azad University, Mahabad Branch, Mahabad, Iran
- Department of Exercise Physiology, Faculty of Physical Education and Sport Sciences, University of Tehran, Tehran, Iran
- Department of Physical Education and Sport Science, Islamic Azad University, Karaj Branch, Karaj, Iran
- Exercise Physiology Department, Islamic Azad University, Central Tehran Branch, Tehran, Iran
- Islamic Azad University Central Tehran Branc


