R Theal, S McLeay, J Gibson, B Lawford, R Mellor
Abstract
Background: Pharmacological management of complex psychological conditions and physical comorbidities in Vietnam veterans can be challenging, particularly when there are multiple prescribing clinicians.
Purpose: To investigate the incidence of psychotropic polypharmacy in a cohort of Australian Vietnam veterans with post-traumatic stress disorder (PTSD).
Methods: Subanalysis of data from a cross-sectional study of Vietnam veterans conducted at Gallipoli Medical Research Foundation from 2014–2015. Relationships between psychotropic polypharmacy and health outcomes were assessed, and anticholinergic burden scores calculated.
Results: Of 160 participants with PTSD, 107 (66.9%) were treated with psychotropic medications, with 53 (33.1%) prescribed two or more. The most common combination was antidepressants with anxiolytics (21.5% of those treated). Polypharmacy was significantly associated with PTSD symptom severity (p<0.01), comorbid depression (p<0.05) and current suicidality (p<0.01). Anticholinergic burden scores ranged from 0–7, with 37.5% of participants classed as medium risk (score = 1–2) and 16.9% as high risk (≥3).
Conclusion: Psychotropic polypharmacy prevalence in Australian Vietnam veterans with PTSD is high. This condition is commonly refractory to pharmacotherapeutic intervention, often leading to trials of combination therapy without supportive scientific evidence. Further research would be beneficial in evaluating potential efficacy versus harm of combination therapies to assist in rationalising prescription of multiple medications.
Conflict of Interest: No conflicts of interest to declare.
Introduction
Post-traumatic stress disorder (PTSD) is a complex mental health condition that can develop after exposure to a traumatic event, characterised by intrusive memories, significant changes in mood and behaviour and avoidance of reminders of the traumatic event.1 Australian guidelines recommend evidence-based trauma-focused psychological treatments (TFPs) as first-line therapy for PTSD, followed by pharmacotherapy as second-line treatment when patients are unable or unwilling to engage in psychotherapy, have severe comorbidities or insufficient response to psychotherapy.2
However, these guidelines are limited for the use of psychotropic medications in treating PTSD. Although selective serotonin reuptake inhibitors (SSRIs) are recommended as the first choice of psychotropic drug class for PTSD treatment, there is insufficient evidence to recommend one SSRI over another or for the indication of other drug classes.2
Where SSRIs are not effective, new generation and older tricyclic antidepressants are recommended as second-line pharmacotherapy, with the addition of an antipsychotic, as adjunctive medication may be indicated when symptoms have not responded adequately.2 However, there is insufficient evidence to recommend antipsychotics in general practice settings.3 Benzodiazepine use in this patient population is associated with harm, and only one drug (paroxetine) is approved for the treatment of PTSD by the Therapeutic Goods Administration (TGA) in Australia.
The absence of compelling evidence for the efficacy of medications and lack of approved options presents a prescribing challenge for clinicians, often further complicated by numerous psychological and physical comorbid conditions. Thus, the likelihood of psychotropic polypharmacy (i.e. concomitant prescription of multiple psychotropic medicines) in patients with PTSD is high, especially when several prescribing clinicians are involved in a patient’s care.
Polypharmacy increases the risk of adverse drug events and drug interactions4 leading to increased morbidity and mortality. In older individuals, psychotropic polypharmacy is further complicated by reduced drug clearance capacity, multiple coexisting pathologies and overall polypharmacy. In one United States (US) study, 50% of veterans over 65 years had at least one prescribing problem such as high- risk prescribing, adverse drug-drug or drug-disease interaction,4 while another found over one-third of veterans with PTSD were on two psychotropic medications, with 10% on three or more.5
The prevalence of any psychotropic polypharmacy in the general Australian population has been reported to be up to 15%, with the most common class combinations including benzodiazepines.6 In Australian veterans, previous studies have reported on the prevalence of antidepressant- related drug interactions7 and analgesic use in veterans with musculoskeletal pain,8 but data on overall psychotropic prescribing for PTSD is lacking. This study aimed to investigate the incidence of psychotropic polypharmacy in a cohort of Australian Vietnam veterans with PTSD, to evaluate the appropriateness of medication prescription based upon current Australian clinical guidelines, and to determine the clinical risk associated with polypharmacy.
Methods
Data were available from the PTSD Initiative study9 performed at Gallipoli Medical Research Foundation (GMRF), Brisbane, Australia, between 2014 and 2015. Participants were male veterans who had served in the Australian or New Zealand armed services in Vietnam during the Vietnam War. Participants were recruited via the veteran mental health unit at Greenslopes Private Hospital, online advertising, print media and word of mouth. All participants provided written informed consent.
PTSD diagnosis was determined by specialist psychiatric evaluation using DSM 5 criteria. Major depressive disorder (MDD), generalised anxiety disorder (GAD), alcohol and substance abuse, and suicide risk were determined using the Mini International Neuropsychiatric Interview (MINI).10
Excessive alcohol consumption and alcohol use disorders were also screened for using the Alcohol Use Disorders Identification Test (AUDIT)11 and general medical screening questionnaires. Sleep disturbances were determined by a structured sleep history questionnaire.12 Daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS).13 Cognitive functioning and impairment were screened with the Montreal Cognitive Assessment (MoCA).14 Diagnosis of comorbid medical conditions were determined through medical screening questionnaires.
Symptoms of PTSD, depression, anxiety and stress were assessed using the Clinician-Administered PTSD Scale for Diagnostic and Statistical Manual of Mental Disorders-5 (CAPS-5)15 and the Depression, Anxiety, Stress Scale-21 (DASS-21).16
Current medication lists were obtained from participants, who were also asked to report the indication for each drug—where known. Medications were coded according to their Anatomical Therapeutic Chemical Classification (ATC) class17 with specific interest in medications used for the nervous system (code N), excluding anaesthetics (N01). Lithium (N05AN) was considered its own class, and to more accurately determine within-class polypharmacy, all benzodiazepines within the hypnotic and sedative class (N05CD) were included with anxiolytics (N05B). Certain cardiovascular-related drugs known to be prescribed off-label for anxiety disorders were also assessed, including beta-blockers (C07A) and prazosin (C02CA01).
Relationships between psychotropic polypharmacy and several health outcomes (PTSD severity, daytime sleepiness, cognitive impairment, depression, stress and anxiety severity, and previous treatment) were determined by Fisher’s exact test or linear regression analysis, where appropriate. Anticholinergic burden scores were calculated as a measure of anticholinergic side-effect risk, based upon medication lists.18
The study was approved by the Greenslopes Research and Ethics Committee (13/53), the Department of Veterans’ Affairs (014/002), The University of Queensland (2014000174), and the Queensland University of Technology (9339361).
Results
Demographics
Of the 299 enrolled participants who underwent psychiatric evaluation (Figure 1), 160 were diagnosed with PTSD. Mean age of participants was 68.5 (SD, 4.2) years, with a mean CAPS-5 score of 15.7 (9.8) (Table 1).
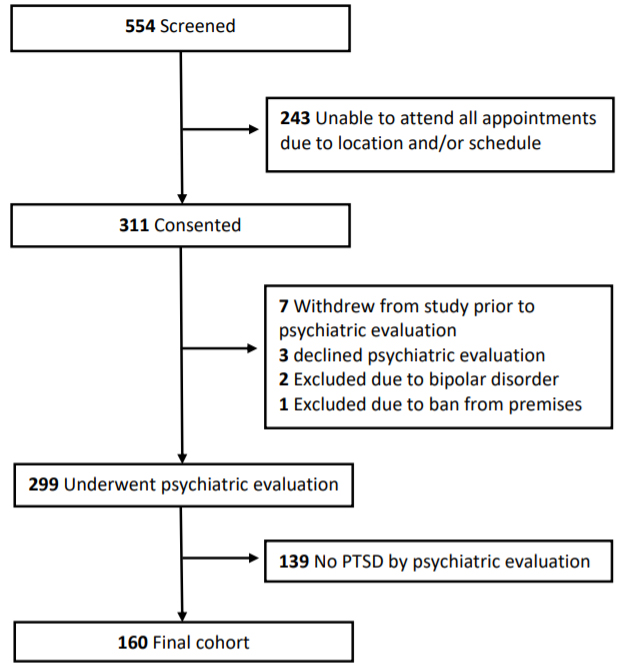
Figure 1. Study Design
Table 1. Participant demographics
| Demographic | Mean ± SD (range), or n (% participants) |
|
|---|---|---|
| Age (years)a | 68.5 ± 4.2 (60-88) | |
| CAPS-5 total symptom scorea | 15.7 ± 9.8 (0-56) | |
| Highest education level | University | 36 (22.5%) |
| Year 11-12 | 34 (21.2%) | |
| Year 10 | 29 (18.1%) | |
| <Year 10 | 27 (16.9%) | |
| Vocational | 32 (20%) | |
| Not reported | 2 (1.2%) | |
| Total number of any medications | 5.9 ± 3.9 (0-16) | |
| Number of participants with >5 medications | 80 (50%) | |
| Number of psychotropic medications | 0 | 53 (33.1%) |
| 1 | 54 (33.8%) | |
| 2 | 32 (20.0%) | |
| 3 | 9 (5.6%) | |
| 4 | 9 (5.6%) | |
| 5 | 3 (1.9%) | |
| Ever sought help fromb | Psychologist | 66 (41.2%) |
| Psychiatrist | 115 (71.9%) | |
| Both psychologist & psychiatrist | 62 (38.8%) | |
| Comorbid | Major depression | 22 (13.8%) |
| Generalised anxiety disorder | 12 (7.5%) | |
| Epilepsy (any past diagnosis) | 6 (3.8%) | |
| Parkinson’s disease | 0 (0%) | |
| Obstructive sleep apnoea | 67 (41.9%) | |
| Regular pain | 86 (53.8%) | |
| Sleep Disturbance | Nightmares | 142 (88.8%) |
| Self-reported poor sleep | 103 (64.4%) | |
| DASS-21 scoresc | Depression score | 6.4 ± 5.0 (0-21) |
| Anxiety score | 6.2 ± 4.5 (0-20) | |
| Stress score | 10.4 ± 4.8 (0-21) | |
| Current suicide risk | 31 (19.4%) | |
| Alcohol use | Abuse | 3 (1.9%) |
| Dependence | 22 (13.8%) | |
| For sleep | 26 (16.2%) | |
| AUDIT score category | Low (0-7) | 86 (53.8%) |
| Risky (8-15) | 55 (34.4%) | |
| High (16-19) | 12 (7.5%) | |
| High, likely dependent (>=20) | 7 (4.4%) | |
| Substance use | Abuse | 0 (0%) |
| Dependence | 1 (0.6%) | |
| MoCAc | 26 ± 2.6 (19-30) | |
| ESS | Total score | 8.9 ± 5.4 (0-24) |
| Score ≥10 (excessively sleepy) | 60 (37.5%) |
Supplementary Table S1. Drug combinations (any polypharmacy)
| Opioids | Antie-pileptics | Anti-parkinson | Anti-psychotics (excluding lithium) | Anxiolytics(includes benzodiazepines) | Hypnotics & Sedatives | Anti-depressant | Psycho-stimulants | Lithium | |
|---|---|---|---|---|---|---|---|---|---|
| Opioids | 1 (0.9%) | 3 (2.8%) | – | 3 (2.8%) | 5 (4.7%) | 2 (1.9%) | 9 (8.4%) | – | – |
| Anti-epileptics | 3 (2.8%) | – | – | 3 (2.8%) | 2 (1.9%) | – | 11 (10.3%) | – | 1 (0.9%) |
| Anti-parkinson | – | – | – | – | 1 (0.9%) | – | 2 (1.9%) | – | – |
| Antipsychotics (excluding lithium) | 3 (2.8%) | 3 (2.8%) | – | – | 5 (4.7%) | 4 (3.7%) | 14 (13.1%) | – | 1 (0.9%) |
| Anxiolytics (including benzodiazepines) | 5 (4.7%) | 2 (1.9%) | 1 (0.9%) | 5 (4.7%) | 3 (2.8%) | 4 (3.7%) | 23 (21.5%) | – | – |
| Hypnotics & Sedatives (excluding benzodiazepines) | 2 (1.9%) | – | – | 4 (3.7%) | 4 (3.7%) | – | 10 (9.3%) | – | – |
| Anti-depressant | 9 (8.4%) | 11 (10.3%) | 2 (1.9%) | 14 (13.1%) | 23 (21.5%) | 10 (9.3%) | 10 (9.3%) | 1 (0.9%) | 1 (0.9%) |
| Psycho-stimulants | – | – | – | – | – | – | 1 (0.9%) | – | – |
| Lithium | – | 1 (0.9%) | – | 1 (0.9%) | – | – | 1 (0.9%) | – | NA |
Participants were prescribed an average number of 5.9 (3–9) medications and had previously sought help for their mental health more frequently from a psychiatrist rather than psychologist (71.9% vs 41.2%; Table 1). Almost half had an AUDIT score in the risky or high categories (46.2%), with 16.2% using alcohol to help get to sleep, and 13.8% diagnosed as currently alcohol dependent, while other substance use was low.
According to the MINI, 13.8% had current, recurrent or melancholic comorbid MDD, and 7.5% had GAD (Table 1). Almost 1 in 5 (19.4%) had a current suicide risk. Over half (53.8%) reported suffering recurring pain, and 41.9% had been previously diagnosed with obstructive sleep apnoea (OSA).
Psychotropic polypharmacy
Of the 160 PTSD participants, 107 (66.9%) were prescribed psychotropic medications, with 53 (33.1%) prescribed two or more (Table 1). Polypharmacy was significantly associated with CAPS total symptom severity score (p<0.01) and comorbid depression (p<0.05), but not comorbid GAD. There was no significant difference in psychotropic polypharmacy rate between those who had sought help from a psychiatrist and those who had not; however, there was an increased odds ratio of 3.0 (95% CI 1.5-6.4) of polypharmacy for those who had previously sought treatment from both a psychologist and psychiatrist.
Antidepressants were the most commonly prescribed psychotropic class (86.0% of those treated), followed by anxiolytics (23.4%; Table 2). Antiepileptics, antipsychotics, opioids, hypnotics and sedatives were all prescribed at similar rates (11.2-15.9%). Of those prescribed antidepressants, almost 70% were taking a single SSRI or serotonin-norepinephrine reuptake inhibitor (SNRI; n = 61). The remaining veterans (n = 17, 18.5%) were prescribed mirtazapine (n =4), amitriptyline (n =3) tricyclic (n =3) or atypical antidepressants (n = 1), monoamine oxidase inhibitors (n =12) or were taking two antidepressants (n = 10, 10.8%)
Table 2. Number of psychotropic medications prescribed within each class
| Drug class | ATC code | n | % Pharmacotherapy treated participants with PTSD (n=107) | (% Total participants with PTSD [n=160]) |
|---|---|---|---|---|
| Antidepressants | N06A | 92 | 86.0% | (57.5%) |
| Hypnotics & Sedatives (excluding benzodiazepines (N05CD)) | N05C | 12 | 11.2% | (7.5%) |
| Antiepileptics | N03A | 17 | 15.9% | (10.6%) |
| Antipsychotics (excluding lithium) | N05A | 16 | 15.0% | (10%) |
| Opioids | N02A | 15 | 14.0% | (9.4%) |
| Anxiolytics (including benzodiazepines (N05CD)) | N05B | 25 | 23.4% | (15.6%) |
| Antiparkinson | N04 | 2 | 1.9% | (1.3% |
| Psychostimulants | N06B | 1 | 0.9% | (0.6%) |
| Dementia | N06D | 1 | 0.9% | (0.6%) |
| Lithium | N05AN | 1 | 0.9% | (0.6%) |
Supplementary Table S2. Two class or within-class polypharmacy (2 prescribed psychotropic medications)
| Opioids | Antie-pileptics | Anti-parkinson | Anti-psychotics (excluding lithium) | Anxiolytics(includes benzodiazepines) | Hypnotics & Sedatives | Anti-depressant | Psycho-stimulants | |
|---|---|---|---|---|---|---|---|---|
| Opioids | – | 1 (0.9%) | – | – | 1 (0.9%) | 1 (0.9%) | 2 (1.9%) | – |
| Anti-epileptics | 1 (0.9%) | – | – | – | – | – | 5 (4.7%) | – |
| Anti-parkinson | – | – | – | – | – | – | 1 (0.9%) | – |
| Anti-psychotics (excluding lithium) | – | – | – | – | – | – | 1 (0.9%) | – |
| Anxiolytics (including benzodiazepines) | 1 (0.9% | – | – | – | – | – | 10 (9.3%) | – |
| Hypnotics & Sedatives | 1 (0.9%) | – | – | – | – | – | 4 (3.7%) | – |
| Anti-depressant | 2 (1.9%) | 5 (4.7%) | 1 (0.9%) | 1 (0.9%) | 10 (9.3%) | 4 (3.7%) | 2 (1.9%) | 1 (0.9%) |
| Psycho-stimulants | – | – | – | – | – | – | 1 (0.9%) | – |
The most commonly prescribed combination of psychotropic medications was antidepressants with anxiolytics (21.5% of those treated for PTSD), followed by antidepressants plus antipsychotics (13.1%), antiepileptics (10.3%), or hypnotics and sedatives (9.3%). Opioids were prescribed with antidepressants in 8.4% of the treated population, and combinations of hypnotics/sedatives with an antipsychotic (3.7%) or anxiolytic (3.7%) (Supplementary Table S1).
Within-class polypharmacy was most common for antidepressants, with 9.3% of those treated prescribed two antidepressants (80% also on an additional class of psychotropic; Supplementary Tables S1 and S2). Table 3 presents all within-class combinations. The most common combinations included an SSRI or TCA with mirtazapine (Supplementary Table S3).
Table 3. Within-class polypharmacy drug combinations
| Class | nTotal | Drug combinations (n) | Indication | Comorbid disease |
|---|---|---|---|---|
| Opioids | 1 | Paracetamol/codeine + oxycodone/ naloxone (1) | Pain + Peripheral neuropathy | GAD, MDD, OSA |
| Antidepressants | 10 | Agomelatine + mianserin (1) | PTSD + PTSD | – |
| Amitryptyline + escitalopram (2) | Sleep + NR | – | ||
| Mirtazapine + desvenlafaxine (3) | NR + NR Dep + Dep |
OSA OSA |
||
| Mirtazapine + duloxetine (1) | NR + NR | OSA | ||
| Mirtazapine + escitalopram (1) | NR + NR | MDD, Suicide risk, OSA | ||
| Mirtazapine + venlafaxine (1) | NR + NR Dep + Dep |
MDD, Suicide risk, OSA Suicide risk, OSA |
||
| NR + NR | GAD, OSA | |||
| Anxiolytics | 1 | Diazepam + oxazepam (1) | NR + Sleep | – |
| Diazepam + flunitrazepam (1) | Sleep + Sleep | Suicide risk, OSA | ||
| Flunitrazepam + buspirone (1) | ||||
| Sleep + NR | OSA |
GAD = Generalised anxiety disorder; MDD = Major depression disorder; NR = not reported; OSA = Obstructive sleep apnoea
Supplementary Table S3. Three or more class or within-class polypharmacy, including a list of drug combinations, presented in order of prevalence
| 3 class or within-class combinations (n=9) | n | Drug combinations | |||
|---|---|---|---|---|---|
| Antidepressant (n=8) | + Anxiolytic (n=5) | + Opioid | 2 | Fluoxetine + diazepam + oxycodone/naloxone; Moclobemide + lorazepam + paracetamol/codeine |
|
| + Anxiolytic | 1 | Escitalopram + diazepam + oxazepam | |||
| + Antipsychotic | 1 | Venlafaxine + oxazepam + seroquel | |||
| + Hypnotic/sed | 1 | Mirtazapine + diazepam + zopiclone | |||
| + Antiepileptic (n=2) | + Opioid | 1 | Duloxetine + perindopril + paracetamol/codeine | ||
| + Antipsychotic | 1 | Fluvoxamine + pregabalin + quetiapine | |||
| + Antidepressant (n=1) | + Antiepileptic | 1 | Escitalopram + mirtazapine + lamotrigine | ||
| Anxiolytic (n=1) | + Anxiolytic | + Antiepileptic | 1 | Diazepam + flunitrazepam + valproate | |
| 4 class or within-class combinations (n=9) | n | Drug combinations | |||
| Antidepressant (n=9) | + Anxiolytic (n=5) | + Antidepressant (n=4) | + Antiepileptic | 1 | Escitalopram + diazepam + amitriptyline + pregabalin |
| + Antiparkinson | 1 | Desvenlafaxine + nitrazepam + mirtazapine + ropinirole | |||
| + Antipsychotic | 1 | Venlafaxine + temazepam + doxepin + quetiapine | |||
| + Hypnotic | 1 | Venlafaxine + diazepam + mirtazepine + zopiclone | |||
| + Anxiolytic | + Opioid | 1 | Sertraline + buspirone + flunitrazepam + buprenorphine | ||
| + Antipsychotic (n=4) | + Hypnotic/sed (n=3) | + Antidepressant | 1 | Escitalopram + quetiapine + zopiclone + amitriptyline | |
| + Anxiolytic | 1 | Escitalopram + risperidone + zopiclone + diazepam | |||
| + Opioid | 1 | Venlafaxine + periciazine + zopiclone + tramadol | |||
| + Lithium | + Antiepileptic | 1 | Desvenlafaxine + quetiapine + lithium + carbamazepine | ||
| 5 class or within-class combinations (n=3) | n | Drug combinations | |||
| Antidepressant + Antipsychotic (n=3) | + Antidepressant (n=2) | + Anxiolytic | + Hypnotic/sed | 1 | Desvenlafaxine + quetiapine + mirtazepine + oxazepam + zopiclone |
| + Opioid | 1 | Mianserin + pericyazine + agomelatine + flunitrazepam + oxycodone | |||
| + Antiepileptic | + Opioid | + Opioid | 1 | Amitriptyline + chlorpromazine + gabapentin + oxycodone/ naloxone + panadeine forte | |
| Class | nTotal Prescribed | Drug | Indication |
|---|---|---|---|
| Antiepileptic | 2 (11.2%) | Valproate (2) | PTSD |
| Antipsychotics | 5 (33.3%) | Pericyazine(1), quetiapine (1) | PTSD |
| Quetiapine (2) | Sleep | ||
| Risperidone (1) | Anxiety/Depression | ||
| Opioids | 1 (6.7%) | Oxycodone/naloxone (1) | Sleep |
| Anxiolytics | 10 (71.4%)a | Alprazolam (1), buspirone (1), diazepam (4), lorazepam (1), oxazepam (3) | Sleep |
a From total participants prescribed benzodiazepines not indicated for sleep
When two psychotropics were prescribed, the most common class combination was an antidepressant with an anxiolytic (9.3%) or antiepileptic (4.7%; Table 4). For psychotropic polypharmacy of ≥three (n=21), the most common combinations included an antidepressant and anxiolytic (13/ 21; 61.9%) or two antidepressants (8/ 21; 31%) (Supplementary Table S3). Antipsychotics were also commonly included in the multi-drug regimens (10/21; 47.6%), and most commonly when ≥4 psychotropics were prescribed.
Indications and off-label use
Drug indications were reported by participants for 56.5% of psychotropic prescriptions, excluding antidepressants. Remaining drug indications were unknown or not reported. Where reported—within antiepileptic, antipsychotic, opioid and anxiolytic drug classes—6.7% to 71.4% of prescriptions were used off-label (Table 4). Sleep difficulties (77.8%) and PTSD (16.7%) were the most common indications for the use of off-label prescriptions.
Eleven (6.9%) participants were taking a beta- blocker and 5 (3.1%) were on prazosin. Although more than half of veterans taking beta-blockers did not report the indication (n=6), all reported hypertension, previous myocardial infarction (MI) or angina, suggesting that beta-blockers were most likely prescribed for cardiovascular dysfunction.
Side effects
No association was found between psychotropic polypharmacy and sleepiness (ESS) or cognition (MoCA) scores (both p>0.05).
Contraindications
Of the 22 participants who were alcohol dependent, 5 (22.7%) were prescribed benzodiazepines (4 used alcohol to get to sleep and had OSA, and 3 were suicidal), and one was on an opioid (tramadol).
Only one participant was considered to have substance dependency, whose medications included buprenorphine for chronic pain. Those who were suicidal were significantly more likely to have psychotropic polypharmacy (p<0.01).
Anticholinergic burden
Anticholinergic burden scores ranged from 0–7, with over half of participants scoring one or more: 37.5% with a medium risk score of 1–2, and 16.9% with a high-risk score of ≥3.
Discussion
In this small cohort of Australian Vietnam veterans with PTSD, the prevalence of psychotropic polypharmacy was over twice that of the general Australian population; 33.1% compared to 15%.6 Additionally, half of the veterans with PTSD were prescribed more than five medications of all classes. The high prevalence of psychotropic (and overall) polypharmacy highlights the complex prescribing challenges for this patient group, who often present with comorbid psychological disorders, chronic pain and sleep disorders. Unsurprisingly, this study demonstrated a significant association between psychotropic polypharmacy and increased PTSD severity, comorbid MDD and increased suicide risk. Pharmacological therapy for PTSD also presents challenges for clinicians due to a lack of drugs approved for the treatment of PTSD, and limited research on concurrent use of multiple drugs.19, 20
Antidepressants were the most common class of medication included in polypharmacy combinations, in contrast to the general Australian population, for whom benzodiazepines are most common.6 Anxiolytics (including benzodiazepines) were most frequently added to antidepressants. Concurrent with Australian and international guidelines, the majority of participants on antidepressants were prescribed first-line or second-line pharmacotherapy recommendations. In some cases, two antidepressants were prescribed; however, these cases were possibly more complex as most were also prescribed additional classes of psychotropic medications. While treatment course was unknown, it is probable that participants taking second-line antidepressants, antipsychotics and multiple classes of psychotropic medications did not respond to first-line PTSD treatment recommendations.
In this study, participants reported experiencing nightmares (88.8%), not sleeping well (64.4%), feeling excessively sleepy (37.5%), and 41.9% had a previous diagnosis of OSA. Gold-standard treatment of PTSD may help improve sleep-related symptoms, but often does not resolve sleep disturbance completely,23 potentially resulting in increased polypharmacy and prescribing off-label drugs. In this cohort, sleep problems were indicated for almost one-third of the total reported psychotropic drug indications. Furthermore, sleep problems were indicated for approximately 70% of reported off-label drug use. Despite this, there is a lack of randomised controlled trials comparing or combining pharmacological and behavioural therapies for insomnia in this population.19
Almost one-quarter of psychotropic pharmacotherapy treated participants reported taking anxiolytics including benzodiazepines, and when duration was reported for benzodiazepine and hypnotic drug treatment for sleep, most reported many years of use (2–42 years), in contrast to current guidelines.2,24 Benzodiazepine use exceeding one month is not recommended,2 and long-term use is associated with physical dependence, increased risk of falls, negative impact on cognitive function and increased risk of dementia.25 Withdrawal from benzodiazepines can also result in serious side effects and requires careful monitoring. In the US, benzodiazepine use is discouraged in veterans with PTSD due to lack of evidence for their efficacy and significant side effects.21 Benzodiazepines have been associated with increased PTSD severity, depression, aggression and poorer outcomes following psychotherapy in patients with PTSD.26
In addition to antipsychotic and benzodiazepine- related adverse effects, drugs including certain antidepressants and opioids contribute to overall anticholinergic burden resulting in an increased risk of cognitive impairment, falls and mortality.18 In our cohort, over half of the participants had a medium or high risk of anticholinergic adverse drug-related events based on the anticholinergic burden score.18 Consideration of anticholinergic burden and other drug-drug or drug-disease interactions is challenging when receiving care from multiple clinicians and is particularly relevant for patients unaware of the indication for which a medication is prescribed. In this cohort, only 55% of patients reported the indication for their prescription, suggesting a lack of patient knowledge surrounding their prescriptions. The Australian Government’s digital health record system allows access to patient health records and prescription history; however, it is not inclusive of all medical records due to significant patient opt-outs and missing historical data. Therefore a thorough medical history is essential prior to prescribing new drugs, particularly benzodiazepines or anticholinergic medications for patients with PTSD; specifically in those with comorbid sleep or substance use disorders.
This is the first study reporting overall psychotropic medication use in Australian Vietnam veterans with PTSD. Inclusion of comorbid diagnoses and actual polypharmacy rates are major strengths of the study, as previous studies using government prescribing data generally have not reported comorbid conditions, make assumptions on medication use based on prescription data and may not capture non-subsidised prescribing. Furthermore, this study provided data on drug indications, demonstrating a significant proportion of off-label drug use.
A limitation of this study was that daily dose information was not captured; therefore, we were unable to use more complex drug interaction risk calculators. Although treatment duration was recorded for some prescriptions, longitudinal prescription data were unavailable, precluding the ability to assess treatment course. Future studies would benefit from collecting more detailed prescription dose and frequency data. Longitudinal studies may also be valuable to identify patterns in pharmacotherapy for treatment-resistant PTSD.
Conclusion
This small cohort of Australian Vietnam veterans with PTSD had a high prevalence of psychotropic and overall polypharmacy despite limited evidence supporting the efficacy of psychotropic medications for the treatment of PTSD. A significant proportion of patients were prescribed medications with little efficacy and associated with harmful outcomes for off-label indications. Further research is required to provide improved prescribing guidelines, particularly in complex, treatment-resistant PTSD cases. Development, assessment and promotion of user- friendly drug or psychological alternatives for PTSD treatment are paramount.
Corresponding Author: R Mellor MellorR@ramsayhealth.com.au
Authors: R Theal1, S McLeay1, J Gibson2, B Lawford2,3,4, R Mellor1
Author Affiliations:
- Gallipoli Medical Research Foundation, Veterans Mental Health Institute
- Greenslopes Private Hospital, Keith Payne Unit
- Queensland University of Technology, Facility of Health and Institute of health and Biomedical Innovation
- University of Queensland Faculty of Medicine and Biomedical Sciences






