C White, MC Reade
Abstract
Many militaries around the world use oral and/or parenteral antibiotics in the pre-hospital environment. This narrative literature review investigated the potential use of pre-hospital antibiotics in the ADF by searching several electronic databases for primary evidence from comparative studies of both prospective and retrospective design. There are concerns that the use of pre-hospital antibiotics may increase individual and population bacterial resistance, impede other pre-hospital tasks and increase the risk of Clostridium difficile and anaphylaxis. Opposing this, there is a known relationship between delay in the administration of antibiotics and mortality due to sepsis; however, the timeline of when antibiotics are required is not concrete. In contrast, there is a known relationship between delay in antibiotic administration and wound infection for penetrating trauma, advocating for pre-hospital antibiotics for penetrating trauma. The use of oral and parenteral antibiotics, such as clindamycin and cefazolin, administered by medical technicians, would benefit individuals with penetrating wounds, and its implementation into the ADF standard practice should be considered.
Key Words: Pre-hospital; Antibiotics; Military medicine; Penetrating wounds; Sepsis
Introduction
Historically, pre-hospital antibiotics have been widely administered, with the use of topical sulphonamide antibiotics common during World War II.1 Changing wounding profiles with the increase in blast injuries and concomitant wound contamination together with improved protection leading to increased survival means that post-injury infections are of increasing importance.2 It is estimated at least a third of all combat casualties will develop infections during their initial hospitalisation.3 In an attempt to combat this, several countries, including the United States of America (USA) and Israel, have developed protocols for pre-hospital antibiotic use. Thus far, this practice has not been adopted by the Australian Defence Force (ADF).
This paper aims to investigate the potential use of pre-hospital antibiotics within the ADF. There are two distinct scenarios in which pre-hospital antibiotics are likely to be administered: sepsis and penetrating/open wounds. This paper intends to detail the benefits and risks of adopting pre-hospital antibiotics into the clinical care continuum and make recommendations on the most appropriate medication.
Methods
The literature review had no language or chronological restrictions with the following electronic databases searched: PubMed/Medline, EMBASE, Cochrane Central Register of Controlled Trials and clinicaltrials.gov. A customised search strategy was built around the MeSH terms and keywords for pre- hospital, antibiotics, sepsis and penetrating wounds. An internet search using the ‘Google Scholar’ search engine with the terms ‘sepsis, ‘penetrating wounds’ and ‘pre-hospital antibiotics’ was also undertaken. Reference lists from full-text articles identified were hand searched for any additional references relevant to the review question.
Pre-hospital antibiotics for sepsis
The Surviving Sepsis Campaign recommends the administration of empirical antibiotics within one hour for septic patients.4, 5 Time to antibiotics has been widely studied in regards to sepsis; however, primarily within the hospital setting. A number of studies, including populations with 20–47% mortality, noted delayed antibiotic administration was associated with increased mortality.6–13 Kumar et al. noted in patients with septic shock that each hour of delay in antibiotic administration over the six hours following the onset of hypotension was associated with an average decrease in survival of 7.6%.6 Studies have demonstrated adverse effects on length of stay,14 acute kidney injury,15 and acute lung injury16 with delayed antibiotics in septic patients.
In a meta-analysis of 16 178 patients across 11 studies, Sterling et al. noted a mortality pooled odds ratio (OR) for patients who received antibiotics three or more hours after triage was 1.16 (95% confidence interval [95% CI] 0.92–1.46; P=0.21) compared to those receiving antibiotics within three hours of triage.17 Authors have argued that most studies within the meta-analyses had significant limitations, including small sample size, arbitrary initial index time point (e.g. emergency department [ED] triage or initial blood culture draw), and indexing of outcome to delay to first antibiotic rather than first appropriate antibiotic.18 Sterling et al. noted in response, the study was intended to evaluate the role of time to antibiotics as a marker of quality of care rather than the impact of time to effective antibiotics on outcomes.19
Since the publication of Sterling et al.’s meta- analysis, Seymour et al. has published two large cohort studies.12, 13 The first studied 49 331 patients with sepsis or septic shock across 149 hospitals, with longer time to antibiotics (OR 1.04 per hour; 95% CI 1.03–1.06; P<0.001) associated with higher risk-adjusted in-hospital mortality.12 Patients who received antibiotics 3–12 hours post-sepsis protocol activation had 14% higher odds of in-hospital death than those who received antibiotics within three hours (OR 1.14; 95% CI 1.06–1.22; P=0.001).
This was replicated in a retrospective study of 35 000 randomly selected ED inpatients in Northern California.20 The second Seymour et al. study of 2683 patients with community-acquired sepsis used various start points to evaluate the effect of antibiotic delay, e.g. time from 911 call, time from paramedic arrival and time from ED arrival.13 Multivariate analysis found the delay in antibiotic administration from paramedic arrival was associated with increased in-hospital mortality (adjusted mortality OR 1.03 [95% CI 1.00–1.05] per 1-hr delay; P<0.01).
The benefits of pre-hospital antibiotics for sepsis may depend on patient population. De Groot et al. noted in a prospective multicentre study based in Dutch EDs that there was no overall association between time to antibiotics and surviving days outside the hospital or mortality.21 Among individuals with less severe sepsis, there was a paradoxically significant increase in surviving days outside the hospital at day 28 associated with delayed (>3 hours) antibiotic administration and attributed to the effect of residual confounding not accounted for in multivariable adjustment for illness severity. The confounding effect of antibiotic indication, in which the most unwell patients receive earlier treatment but conversely those with occult infection (and hence worse prognosis) might have antibiotic treatment delayed, plagues all observational studies of this question.
A more recent study retrospectively reviewed 308 patients with septic shock treated by a French Mobile Intensive Care Unit, with 98 (32%) receiving predominantly third generation cephalosporins (cefotaxime (47; 67%) or ceftriaxone (27; 36%)).22 The study found a significant association between pre-hospital antibiotic therapy and 30-day mortality with an adjusted hazard ratio of 0.56 (95% CI 0.35–0.90; P=0.01). Individuals who received pre- hospital antibiotics also had shorter hospital stays (7 vs 17 days; P<0.001). This was despite individuals receiving pre-hospital antibiotics having a higher mean age (71 vs 69 years; P=0.001), mean lactate (5.1 ± 3.8 vs 5.9 ± 3.2 mmol/L; P=0.008), SAPS2 score (51 ± 24 vs 53 ± 30; P<0.001), and a greater proportion of individuals with chronic cardiac (43% vs 7%; P=0.006) and renal failure (29% vs 5%; P=0.027). Statistically significant differences were also noted in SOFA scores and Glasgow Coma Scale scores, although the clinical significance is doubtful. Randomised controlled trials (RCTs) are the only method to minimise the effect of both observed and potentially unobserved confounding, which makes them superior to observational study designs. Randomising to delay in appropriate antibiotics would be an unethical and spurious question. That being so, as pre-hospital antibiotics are not standard care and might be associated with risk as well as benefit, there could be equipoise to conduct a trial of this question. An early small RCT randomised 198 patients with septic shock to pre-hospital broad- spectrum intravenous antimicrobial therapy and intravenous fluid therapy or intravenous fluid only.23 There was a significantly shorter mean intensive care unit (ICU) stay in the pre-hospital antibiotic cohort (6.8 ± 2.1 days vs 11.2 ± 5.2 days; P=0.001). The 28- day mortality rate was also significantly reduced with the pre-hospital antibiotic cohort (42.4% vs 56.7%; P=0.049; OR = 0.56; 95% CI 0.32–1.00). These results should be interpreted with caution as this data, to date, has only been presented in conference abstract form.
In contrast, the PHANTASi Trial evaluated the use of pre-hospital antibiotics for sepsis in 2672 patients, of whom 1535 received antibiotics a median of 26 minutes (Inter-Quartile Range [IQR] 19–34 minutes) prior to arriving at the ED.24 Those in the control group had a median time to antibiotics of 70 minutes (IQR 36–128 minutes) after ED arrival. Of note, 99% of those administered pre-hospital antibiotics had blood cultures prior to antibiotic administration. This is important in that it allows the targeting of step- down antibiotics with narrowing of the antibiotic spectrum in hospital care. There were no significant differences in ICU admissions, length of ICU stay, length of hospital stay, 28-day mortality and 90-day mortality between those who did and did not receive pre-hospital antibiotics. Patients given pre-hospital antibiotics had a significantly lower proportion of 28-day re-admissions (7% vs 10%; P=0.0004). The PHANTASi cohort was relatively clinically well, with only 3–4% having septic shock and approximately 10% of patients requiring ICU admission. The power analysis assumed a 40% baseline mortality rate for sepsis, which the PHANTASi authors based on ICU studies of patients with severe sepsis and septic shock. This was considerably higher than the 7.97% (213 deaths in 2672 patients) 28-day mortality rate in the PHANTASi cohort, which suggests the study might be underpowered to find a 28-day mortality difference. Even among those with septic shock, mortality was 28.15% (29 deaths in 103 patients). Consequently, the study’s failure to observe effects in the most important outcomes might have been because the patients included were not unwell enough to benefit.
Another potential cause for the lack of difference in mortality among patients with severe sepsis and/ or septic shock in the PHANTASi Trial was that the difference in time to antibiotics may not be as profound among sicker patients. Sicker patients may have been transported to hospital more expeditiously and may be assessed and treated quicker within the ED, thus minimising the difference between the two groups. The PHANTASi trail authors note (in the Netherlands) that response times and arrival to ED times are relatively short. Therefore, it would be reasonable to assume that sicker patients would receive more expeditious treatment and potential smaller study arm time differences.
Other issues noted with the PHANTASi study include that general practitioners had referred 73% of patients, and 22% were already on oral antibiotics. It is unclear what this may mean, but it is at variance to the military context where the pre-hospital team is often proximal to the point of injury and the first form of medical care that a patient receives. Additionally, the study was conducted in the Netherlands, which is relatively geographically compact in contrast to the military and Australian context, where there may be considerable distances and/or time to medical care. There was also a difference in the proportion of individuals receiving pre-hospital intravenous fluids, with 64% of the intervention and 37% of the control group receiving pre-hospital intravenous fluids. This probably reflects the intravenous access obtained to allow administration of intravenous antibiotics in the intervention cohort.
In summary, it can be seen that the evidence for pre-hospital antibiotics for septic patients while supportive is by no means definitive. This is not entirely unexpected given the disparate causes and complex heterogeneity of sepsis and the potential for different types of sepsis to behave differently. Further, the military pre-hospital environment is profoundly different from that of the civilian, which must be considered in future studies. There are several pre-hospital trials currently occurring, and this should provide further clarity.
Pre-hospital antibiotics for penetrating wounds
It is common civilian practice to administer antibiotic prophylaxis to patients with open fractures. The Gustillo classification is used to grade soft-tissue injury associated with open fractures. Gustillo Grade I and II injuries (wounds less than 10 cm in size, no more than moderate contamination, and no periosteal stripping) are usually treated with a first-generation cephalosporin (such as cephazolin) until 24 hours after wound closure.25 Grade III wounds are, at times, additionally treated with an aminoglycoside, fluoroquinolone, third–fourth-generation cephalosporin or penicillin, depending on potential contamination. Several studies have demonstrated that the earlier antibiotics are administered for open fractures, the better the individual’s outcomes.26–28 Preclinical animal models have demonstrated reduced wound colonisation, infection rates and osteomyelitis with antibiotic administration closer to time of injury.29–37 For penetrating abdominal trauma, prophylactic antibiotics are widely utilised and recommended,38 and several RCT meta-analyses demonstrate the utility of prophylactic antibiotics for penetrating thoracic wounds.39, 40 In 1972, Fullen et al. noted a 7–11% post-surgical infection rate with preoperative antibiotics, a 33–57% infection rate with intraoperative antibiotic administration and a 30–70% infection rate with only post-operative antibiotic administration.41
In the civilian pre-hospital arena, Thomas et al. prospectively evaluated pre-hospital antibiotics for open fractures in 138 consecutive trauma cases transported by eight US helicopter-based emergency medicine service programs.42 The time from injury to antibiotics was 30 minutes shorter (47 minutes vs 77 minutes; P<0.0001) when pre-hospital antibiotics were administered. There was no statistical difference between pre-hospital and hospital antibiotic administration for a composite endpoint (infection/ non-union). However, this was likely due to the small sample size with only one adverse outcome in the pre-hospital antibiotic cohort.
Evidence from military studies using hospital-based cohorts suggests that early antibiotic administration for open combat wounds reduces infection rates. During the Falklands Islands Campaign, it was noted that no septic complications occurred in soft-tissue extremity injuries when antibiotics were administered within three hours of injury.43 Gerhardt et al. studied 53 patients injured in Central Iraq, with 43 (81%) receiving systemic antibiotic prophylaxis.44 Despite no difference in wound mechanisms and anatomical locations, those with antibiotics were significantly less likely to develop an infection within 48 hours (7% vs 40%; OR 0.11 [95% CI 0.02–0.57]), with a number needed to prevent infection within 48 hours of three (95% CI 2–14).
Studies of pre-hospital antibiotic use in the military environment and its effects on open combat wounds are limited. Early studies with narrow population sizes noted no benefit in preventing infectious complications between those receiving and not receiving pre-hospital antibiotics.45, 46 The most comprehensive study reviewed the US Department of Defense Trauma Registry from January 2007 to August 2016 and identified 15 114 individuals with gunshot wounds (GSWs), traumatic amputations or open-fractures proximal to the digits.47 No difference in survival until discharge between those receiving and not receiving pre-hospital antibiotics was noted among patients with amputations (93.9% vs 90.7%, P=0.271) or open fractures (96.8% vs 95.9%, P=0.368). Among patients with GSWs there was a significantly higher survival rate among those receiving pre-hospital antibiotics (96.2% vs 92.8%, P<0.001), which persisted in multivariable regression analysis (OR 1.61; 95% CI 1.09–2.38).
Taken together the general consensus is that for traumatic penetrating wounds there is a reasonable body of evidence that early and pre-hospital antibiotics reduce morbidity and mortality. In particular short course and single agent regimens have a role in preventing adverse outcomes in open fractures (25).
Risks of pre-hospital antibiotic use
The addition of any intervention brings with it the potential for additional risks to the patient. In the case of pre-hospital antibiotics, it could include allergic or adverse drug reactions, increased antibiotic resistance and inappropriate antibiotic use.
Anaphylaxis and adverse drug reactions
With the more widespread use of any therapeutic agent, there is the risk of inducing allergic reactions to the agent in question. There is also the risk of other adverse drug reactions, which was demonstrated by the replacement of gatifloxacin with moxifloxacin in the Tactical Care of the Combat Casualty (TCCC) algorithm due to concerns associated with dysglycaemia. Further, there is the risk of the development of other adverse effects, including Clostridium difficle infections. Within the Australian population, fatal anaphylaxis is a rare, albeit devastating, occurrence.48 Anaphylaxis to beta-lactam antibiotics occurs in approximately 0.001% of parenteral exposures and 0.0005% of oral exposures,49, 50 with penicillin anaphylaxis appearing to be declining in frequency.51 Evidence from the use of a single dose of prophylactic antibiotics prior to dental surgery for patients at risk of infective endocarditis has identified a crude anaphylaxis rate of one in 56 878 dental procedures with single-dose amoxicillin prophylaxis.52 That being so, no fatal anaphylaxis cases were reported.
There were no serious adverse events in those receiving ceftriaxone, such as anaphylactic shock, in the PHANTASi Trial of pre-hospital antibiotics for sepsis.24 Seven mild allergic reactions occurred, but none were attributed to ceftriaxone. In a cohort of 70 individuals with open wounds with a potential underlying fracture, Lack et al. identified eight individuals (11.43%) with a penicillin allergy who were excluded from receiving intravenous cefazolin.53 Three patients were identified to have antibiotic allergies (unknown which antibiotic) during their hospital stay, although all three had received 1–2 grams of intravenous cefazolin pre-hospital, without an adverse event.
Inappropriate antibiotic use
Pre-hospital antibiotic use is constrained by logistics that limit the choices available. This has significant potential implications with survival and morbidity benefits only likely to occur when appropriate pre- hospital antibiotics are given. This can be seen in part by the disparate literature surrounding empirical antibiotic use in sepsis.
There is a risk that using an inappropriate antibiotic in the pre-hospital environment may delay appropriate antibiotic treatment. This is not without consequences; Vazquez-Guillamet et al. demonstrated that among septic ED patients, there was a significant increase in mortality when the initial antibiotic chosen was inappropriate (20.5% vs 47.5%; P< 0.001 OR 3.4 [95% CI 2.8–4.1]). This has been reflected in other studies.54, 55 This can be mitigated by careful selection of the pre-hospital antibiotic to the operating environment and injuries expected to be encountered. There will also typically be a change from the pre-hospital to hospital antibiotic. For example, in the PHANTASi Trial, most patients were continued on amoxicillin-clavulanic acid or ciprofloxacin, not ceftriaxone, when they reached hospital.24 This mimics standard practice with the switch from parenteral to oral antibiotics and broad to narrow-spectrum antibiotics when the clinical picture becomes clearer.
There are also potential issues with adherence to antibiotic guidelines, particularly in the dynamic tactical pre-hospital environment. This is not unexpected as even in the relatively controlled operating theatre environment, approximately 7% of individuals with orthopaedic trauma do not receive appropriate preoperative antibiotics.56 Lloyd et al. evaluated receipt of recommended antimicrobials within 48 hours of injury in 1106 military personnel, finding 74% received antimicrobial prophylaxis within 48 hours of injury.57 Of note, the proportion receiving prophylaxis meeting the guidelines varied from 35–80% depending on the injury. Similar findings were noted by Tribble et al. in a cohort of trauma admissions to Landstuhl Regional Medical Centre, with 75% of trauma patients receiving antibiotic prophylaxis.58
Focusing on the pre-hospital environment, Naylor et al., on review of the US Department of Defence Trauma Register from January 2007 to August 2016, noted the proportion of individuals with combat wounds receiving pre-hospital antibiotic prophylaxis was as low as 9.8%.47 Schauer et al. noted among 50 individuals wounded in Afghanistan, 54% were administered antibiotics, although only 11.1% of the antibiotics were within TCCC guidelines.59 This has been reflected by some,46 although others have noted greater compliance with guidelines, albeit with lower proportions of individuals receiving antibiotics.60 Of note, Schauer et al. found that most individuals seen by a medical officer received antibiotics (73.4%) although only 2.4% of those antibiotics were within the TCCC guidelines.59 In contrast, 20.7% of individuals seen by a medical technician received antibiotics; however, there was a greater (68.6%) concordance with guidelines. Among other populations, higher proportions of patients were noted to have pre-hospital antibiotic prophylaxis; Naylor et al. noting 80% of paediatric trauma patients admitted to Role Three or Forward Surgical Team facilities had received pre-hospital antibiotics.61,62 which can supersede antibiotic administration. The impetus to administer antibiotics may displace or delay some of these interventions, which may be inappropriate. Among those not receiving pre- hospital antibiotics, Benov et al. found there were a greater number of lifesaving interventions required, more hostile battlefield environments and longer evacuation distances.60 Others have also noted greater composite Injury Severity Scores among those not receiving pre-hospital antibiotics.47 Interestingly, civilian studies have noted that longer evacuation times (greater than 30 minutes) increased the chance of receiving pre-hospital antibiotics (29.1% vs 65.8%; P<0.001).53
Microbial resistance
Increasing antibiotic use is widely accepted to be a key contributor to the development of antibiotic resistance. Khalil et al. demonstrated that a single 2 gram amoxicillin dose can significantly alter the oral microbiome and induce a significant selection of resistant microorganisms.63 Studies have also demonstrated increased microbial resistance following antibiotic exposure and increased resistance with increased antibiotic consumption.64–68 Lloyd et al. reviewed individuals with open extremity soft-tissue injuries or open fractures sustained in Iraq and Afghanistan (2009–2014) that required transfer to USA hospitals.69, 70 A higher proportion of fluoroquinolone and/or aminoglycoside resistant gram-negative organisms were found in patients who received expanded gram-negative antibiotic coverage (Soft-tissue injuries 35% vs 19%, p < 0.001; open fractures 49% vs 40%, P<0.001).
There is minimal data regarding the effect of pre-hospital antibiotics on microbial resistance, although there are some potential indirect markers. In the PHANTASi Trial, positive urine cultures were less frequent among those given antibiotics prior to hospital arrival (25 [25%] of 1048 vs 295 [37%] of 801; P<0·0001), which the authors attributed to pre-hospital antibiotics.24 More definitively, Murray et al. retrospectively reviewed the outcomes of 211 causalities up to 30 days post-injury, with 28 (13.3%) subsequently found to be infected with gram-negative bacteria with pre-hospital administration of a single- dose broad-spectrum antibiotic not altering infection or colonisation rates.46
Antibiotic options
The ideal pre-hospital antibiotic would be well tolerated, stable across a range of environmental conditions, easily administered in a dynamic environment, active against a wide variety of potential microorganisms and have a low risk of adverse effects and microbial resistance induction. Furthermore, the antibiotic would be already available within the ADF formulary or at least within widespread Australian clinical practice.
Pathogens isolated from traumatic war wounds include a mixture of gram-positive and gram- negative organisms, notably Enterococcus species, Staphylococcus aureus, Acinetobacter calcoaceticus– baumannii complex, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae.3, 45, 71–76 The microbiology of traumatic wounds vary as casualties pass through the military medical system from the combat theatre, interim facilities, to final medical treatment facilities.77, 78 In regards to point of injury contamination, Murray et al. cultured 49 US military members with 61 separate traumatic wounds at initial presentation to a combat support hospital in Iraq.79 Thirty wounds (49%) had positive bacterial cultures, with 40 different bacteria identified. Of note, 18 casualties (20 wounds) had pre-hospital irrigation and/or antibiotic therapy; of these, 6 wounds (30%) had positive cultures with 9 different bacterial isolates. Of the 41 wounds from 31 patients who had received no pre-hospital irrigation/antibiotics, 24 wounds (58%) grew 31 different bacteria. Gram- positive bacteria (93%), mostly skin-commensal bacteria, e.g. coagulase-negative Staphylococcus and Staphylococcus aureus, were the predominant organisms identified. Only three gram-negative bacteria were detected, none of which were considered to have significant antimicrobial resistance. The only resistant bacteria recovered were two cultures of methicillin-resistant Staphylococcus aureus. The authors concluded that the use of broad-spectrum antibiotics with efficacy against more resistant, gram- negative bacteria, e.g. Pseudomonas aeruginosa and Acinetobacter, is unnecessary in early wound management. This was reinforced by Lloyd et al. who noted in individuals with open extremity fractures that the use of expanded gram-negative coverage (e.g. fluoroquinolones or aminoglycosides) led to a small reduction in soft-tissue and skin infections but did not decrease the risk of osteomyelitis and led to an increase in antibiotic-resistant organisms.70 A further study by Lloyd et al. of individuals with open extremity soft-tissue injuries noted no significant difference between narrow and expanded gram- negative antibiotic regimes in extremity skin and soft-tissue infection, hospitalisation duration, mortality, number of operations and non-extremity infections.69 Patients that received expanded gram- negative antibiotic regimes were significantly more likely to culture gram-negative organisms resistant to expanded gram-negative antibiotics. Consequently, Lloyd et al. recommended using cefazolin or clindamycin for open fracture wound empirical treatment, which is reflected in the guidelines for the Prevention of Infections Associated with Combat- Related Injuries.80
A key consideration in the use of pre-hospital antibiotics is how the antibiotics are administered, namely oral versus parenteral. The oral route can be easily taken at point of injury, particularly if issued to individual service members. Furthermore, there is decreased time and logistic burden of reconstituting and administered parenteral medication when oral medication is used. Oral medications also allow medical personnel to carry multiple doses in a single foil packet. This must be balanced by the potential inability or inadvisability of oral medication in some wounding scenarios. If issued to all personnel, there is also the potential for oral medication to be misused for incorrect indications, thereby delaying appropriate care and leaving the individual without medication when it is required. Taken together, an oral preparation would theoretically increase the likelihood of an injured individual receiving the medication but has the potential for misadministration/use.
Various militaries use a range of pre-hospital or early medical antibiotic options for the treatment of penetrating combat wounds (Table 1). Militaries from the Federal Republic of Germany, the USA and Israel use antibiotics in the pre-hospital environment, while the United Kingdom and France advocate antibiotic prophylaxis upon arrival at medical facilities. The USA and several other militaries use the TCCC guidelines that recommend a combination of oral and parenteral pre-hospital antibiotics for traumatic wounds.
The TCCC program advocates for the Combat Wound Medication Pack (CWMP) that consists of paracetamol, a non-steroidal anti-inflammatory drug and an oral antibiotic. A fourth-generation fluoroquinolone (400 mg moxifloxacin) choice was based on the range of activity against pathogens expected to be encountered in military environments, relatively low side-effect profiles, favourable oral pharmacokinetics and once- daily dosing options.81 Naylor et al. reviewed the USA Department of Defence Trauma Registry for 11 655 US service members with TCCC indications for CWMP administration between January 2007 and August 2016, finding 84 (0.72%) received pre- hospital oral antibiotics.82 Recipients of the CWMP were less likely to have an extremity injury, more likely to be in Afghanistan (versus Iraq) and have a lower composite injury score. Other studies of special forces units, namely the 75th Ranger Regiment of the US Army, have noted higher utilisation in the region of 19–21%.46, 83 A key limiting factor in the use of CWMP has been postulated to be logistical issues.84,85 Consequently, Naylor et al. recommended that for the wider military that CWMP be supplied to medics rather than individuals to minimise some of the issues associated with appropriate administration and logistical supply.
Table 1. Military pre-hospital antibiotic recommendations
| Country / Organisation | Medication recommendations |
|---|---|
| United States of America Canada |
Oral
Parenteral
|
| Israel Armed Forces |
2 gm IV ceftriaxone Abdominal / Head injuries
|
| German Armed Forces |
Oral
Parenteral
|
| French Armed Forces |
No prehospital guidelines but parenteral prophylaxis on arrival at Medical treatment facility with: 2 gm IV amoxicillin + clavulanic acid OR 600 mg IV clindamycin |
| United Kingdom Armed Forces |
No prehospital guidelines but parenteral prophylaxis on arrival at Medical treatment facility with: Extremity injuries
Hollow viscera injuries
|
Adapted from (91–94).
For parenteral pre-hospital antibiotics, TCCC recommends single-dose ertapenem 1 gm IV or IM if penetrating abdominal injury, shock or unable to tolerate PO medications. Recommended alternate agents include cefotetan 2 gm IV or IM 12 hourly or a single dose of levofloxacin 500 mg PO. Schaeur et al. noted no documented cefotetan use in their review of individuals wounded in Afghanistan, despite its recommendation as an alternative agent.59 They postulated several reasons for this, including lack of awareness, ready ertapenem availability, limited cefotetan availability and higher cefotetan cost.
Moxifloxacin is used to a limited degree in Australian civilian practice, primarily for indications such as community-acquired pneumonia, chronic bronchitis and sinusitis. However, fluoroquinolone resistance has led to recommendations against its use as a first-line agent.86 Ertapenem is a carbapenem related to meropenem, typically reserved for life-threatening infections due to organisms not susceptible to narrower-spectrum agents. In Australian public hospital practice, its use typically requires specialist infectious diseases physician authorisation based on microbiological cultures.
The International Council of the Red Cross (ICRC) recommends that all patients with penetrating wounds receive five million units of benzylpenicillin IV on admission with alternatives in the case of penicillin allergy being erythromycin, chloramphenicol or a cephalosporin.87, 88 The use of penicillin is based on the knowledge that ‘Streptococcus pyogenes, Clostridium welchii and Clostridium tetani are always sensitive to it’.87 The ICRC provides further guidance for a number of additional scenarios to substitute or add additional antibiotics (Table 2).89
The Primary Clinical Care Manual (PCCM) that governs much of the ADF Medical Technician’s practice provides the means for medical technicians to administer antibiotics under remote supervision of a nursing practitioner or medical officer.90 For sepsis, the PCCM recommends the administration of gentamicin plus flucloxacillin with the addition of vancomycin for methicillin-resistant Staphylococcus aureus and ceftriaxone for suspected meningitis (Table 3). The PCCM does not provide a specific recommendation for penetrating traumatic wound antibiotic prophylaxis, apart from some situations (Table 4), suggesting the choice is at the supervising medical officer’s discretion. The electronic Therapeutic Guidelines (eTG), which guide many medical officers’ practice, for the most part, reflect the PCCM, although there are several differences (Table 5).91
Table 2. International Committee of the Red Cross Antibiotic Prophylaxis Protocol for Adults with Weapon Wounds
| Injury | Recommendation |
|---|---|
| Penetrating craniocerebral wounds |
|
| Eye and maxillofacial wounds affecting a cavity (nasal, oral and/or sinus) |
|
| Eye and maxillofacial wounds not affecting a cavity (nasal, oral and/or sinus) |
|
| Surgery or delayed primary closure on minor soft-tissue wounds |
|
| Chest drain placement for haemothorax |
|
| Amputations, open fractures or major soft-tissue wounds | Less than 72 hours from point of injury
Greater than 72 hours from point of injury
|
| Limb injuries from antipersonnel mines |
|
| Abdominal wounds |
|
Adapted from International Committee of the Red Cross Anaesthesia Handbook (88).
Potential pre-hospital antibiotic in the Australian Defence Force
The summation of the above would be that there is a reasonable body of evidence that pre-hospital prophylactic antibiotics reduce wound infection and mortality in penetrating trauma without an appreciable incidence of adverse events. There are a number of opportunities within the pre-hospital continuum to administer antibiotics, ranging from individual issue to provision as part of the evacuation process (Figure 1). The choice of antibiotic and administration protocol is likely to influence when pre-hospital antibiotics can be administered.
A cephalosporin would avoid some issues associated with adverse drug reactions to penicillins. It would also concur with established clinical guidelines, with cefazolin widely utilised in the preoperative setting due to its narrow spectrum of action that still covers the bacteria commonly encountered in surgery and post-operative infection for penetrating wounds.57, 80, 89 Ceftriaxone is already utilised in the PCCM for several clinical indications, including bite wounds, orbital cellulitis, meningitis, sexually transmitted infections, pyelonephritis, urosepsis and epiglottitis.90 Further, ceftriaxone is used by several Australian ambulance services in the pre- hospital environment. Consequently, ADF medical technicians are likely to have already been exposed to and administered the medication. Ceftriaxone also has the added benefit of once-daily dosing and being able to be administered by the intravenous and intramuscular route. Consequently, a first- generation cephalosporin, such as 2 gm cefazolin IV/IM 8 hourly, would be recommended for post- traumatic wound prophylaxis (Table 6). For sepsis, a third-generation cephalosporin, such as 2 gm ceftriaxone IV/IM 12 hourly, could recommend for most parts of the world, unless there are local reasons to modify this, e.g. if melioidosis was a substantial risk, 2 gm ceftazidime IV/IM would be a reasonable choice.
Table 3. Primary Clinical Care Manual Antibiotic Guidelines for Empirical Sepsis and Meningitis Treatment
| Empirical sepsis treatment | |
| Children | |
| < 2 months |
If MRSA risk and > 1 month old ADD 15 mg/kg ≤ 750 mg IV vancomycin |
| 2 months to 16 years |
If MRSA risk and > 1 month old ADD 15 mg/kg ≤ 750 mg IV vancomycin |
| Child with septic shock or critical illness | REPLACE above with:
|
| Adults (> 16 years old) | |
If MRSA risk ADD 30 mg/kg ABW loading dose IV vancomycin |
|
| Additional considerations all ages | |
| If meningitis cannot be excluded ADD 50 mg/kg ≤2 gm IV/IM ceftriaxone | |
| Empirical meningitis treatment | |
| Neonates and infants < 2 months |
|
| Children ≥ 2 months and adults |
If critically ill immunocompetent child ≥ 2 months may ADD Gentamicin PLUS Vancomycin as per |
Note: IBW: Ideal body weight; ABW: Actual body weight; AdjBW: Adjusted body weight.
Adapted from 10th Edition Primary Clinical Care Manual (89).
Figure 1. Australian Defence Force pre-hospital antibiotic administration points
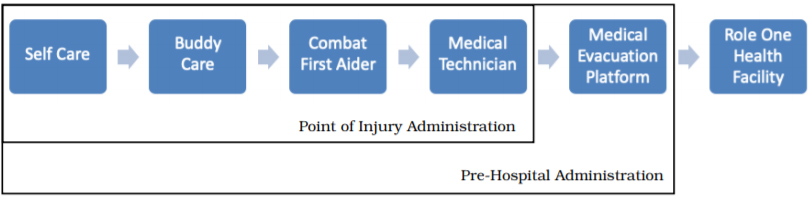
Table 4. Primary Clinical Care Manual Antibiotic Guidelines for Specific Circumstances
| Injury | Recommendation |
|---|---|
| Traumatic jaw injury |
|
| Compound or basal skull fracture | As per meningitis pathway detailed in Table 3 |
| Human (toothknuckle) and animal bites | 875 mg + 125 mg PO amoxicillin + clavulanic acid 12 hourly If lack of adherence is anticipated or delay in commencing oral antibiotics treat with:
if allergic to penicillin, treat with:
|
Adapted from 10th Edition Primary Clinical Care Manual (89).
Table 5. Electronic Therapeutic Guidelines for Antibiotic Prophylaxis
| Injury | Recommendation |
|---|---|
| Open fracture | 2 gm IV cefazolin 8 hourly If water immersion substitute cefazolin with:
If severe / heavily contaminated:
If severe penicillin hypersensitivity and can tolerate oral medication
If water immersion, severe penicillin hypersensitivity and can tolerate oral medication:
|
| Human (toothknuckle) and animal bites | 875 mg + 125 mg PO amoxicillin + clavulanate 12 hourly for three days If lack of adherence is anticipated or delay in commencing oral antibiotics treat with:
If unable to tolerate oral mediation
If allergic to penicillin, or at increased risk of methicillin-resistant Staphylococcus aureus infection:
|
| Penetrating eye injury | 400 mg PO moxifloxacin daily for 5–7 days OR 750 mg PO ciprofloxacin 12 hourly for 5–7 days |
Adapted from electronic Therapeutic Guidelines (90).
Table 6. Proposed Australian Defence Force Prehospital Antibiotic Guideline
| Situation | Recommendation |
|---|---|
| Sepsis / Septic shock | Intravenous/intramuscular agent
|
| Penetrating trauma / Open wound | Oral agent
|
Regarding the provision of an oral antibiotic or equivalent to the CWMP to ADF members, the situation is less clear. The US Armed Forces experience has demonstrated a relatively low uptake of the CWMP outside specialised units. Even with uptake, there is still relatively low utilisation of the medication outside special forces units. In their most recent pre-hospital antibiotic guidelines, the Israeli Defence Force removed oral antibiotics from their algorithm.92 The issues surrounding antibiotic choice and logistic supply are likely to render pre- hospital oral antibiotics an option in development for individual issue. There remains the option to equip appropriately trained medical personal with oral antibiotics to provide to injured service members, which would avoid a number of the associated logistical issues.
The decision regarding the most appropriate oral antibiotic is less clear. As noted in Table 1, other militaries use moxifloxacin, levofloxacin, ciprofloxacin or clindamycin as their oral antibiotics. Fluoroquinolones are not recommended for parenteral early post-traumatic antibiotics; thus, the assumption would be that the expanded spectrum would also not be required for oral pre-hospital medication. Clindamycin is the alternative agent in the German Armed Forces,93 which concurs with the eTG guidelines for individuals with open fractures and penicillin anaphylaxis.91 The German Armed Forces recommend a 600 mg dose,93 while the eTG guidelines recommend 450 mg doses three times a day for open fractures in individuals with penicillin anaphylaxis.91 Australian blister pack sizes of 24 and 200 tablets are available; thus, a blister pack of 24 tablets would provide eight 450 mg or six 600 mg doses in a relatively small and easily administered package. The avoidance of penicillin allergy risks with clindamycin is also beneficial if individual antibiotics issue is to be considered.
The future
There remain concerns that pre-hospital antibiotics may lead to inappropriate antibiotic choices, increase bacterial resistance in individuals and the population, distract from more useful tasks, and increased risk of adverse drug reactions. Opposing this, there is a known relationship between delay in antibiotic administration and mortality due to sepsis; however, the timeline of when antibiotics are required is not concrete. This suggests that further trials are required for pre-hospital use of antibiotics for sepsis, particularly regarding its applicability to the military context. In contrast, there is a known relationship between delayed antibiotic administration and wound infection for penetrating trauma; advocating for the use of pre-hospital antibiotics for penetrating trauma. Taken together, this would suggest the use of oral and parenteral antibiotics, such as clindamycin and cefazolin, administered by medical technicians would benefit individuals with penetrating wounds and its implementation into the ADF standard of practice should be considered.
Corresponding Author: Christopher White christopher.white14@defence.gov.au
Authors: C White1, M Reade2,3
Author Affiliations:
- Royal Australian Air Force
- Joint Health Command, Department of Defence
- The University of Queensland, Faculty of Medicine



