ABSTRACT
ONLY ONE VACCINE AGAINST JAPANESE ENCEPHALITIS is available for use in Australia. Other vaccines in Asia have supplanted this vaccine. Some vaccines used in Asia, however, would not be acceptable in Australia. A number of candidates are in clinical development based on more efficient platforms and cleaner production lines. The ADF is
involved in clinical trials to ensure earliest availability and applicability to Australian Service personnel.
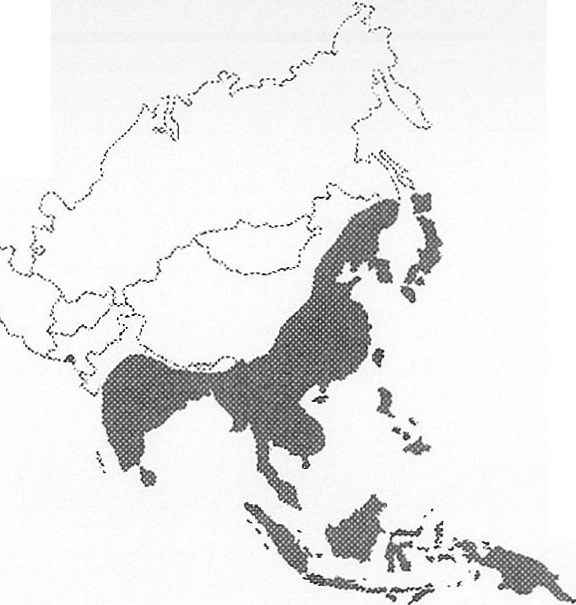
MAP 1: Distribution of Japanese Encephalitis in Asia 1970-1998
INTRODUCTION
FIGURE 1: The vector for Japanese encephalitis virus (Image: Culex mosquitoes, Arboviral Encephalitides, www.cdc.gov ,last updated 13 July 2001).
Japanese encephalitis (JE) is the leading cause of viral encephalitis in Asia with WHO estimates exceeding
70,000 cases annually despite reporting being incomplete. Approximately a third of clinical cases will die and
about one half will have residual neurological sequelae.2 Nevertheless, the chance of contracting travelling in Asia is around 1 in a million’. The distribution of the virus has extended to include Australia, where it is now identified by the US Centers for Disease Control as seasonally endemic.
THE HISTORY OF EXISTING VACCINES
With the rising problem of Japanese Encephalitis causing non-battle casualties in the Pacific Theatre after
1941, Major Albert Sabin prepared an inactivated JE vaccine in mouse brains for use by US Service personnel. In the post-War reconstruction of Japanese industry, several JE vaccine candidates were produced by various agencies. These were generally derived from two main strain groups isolated 30 years before, the Nakayama strain and the JaGAr group. The immune genicity derived from the latter was inadequate despite various growth media, including mouse brains and cell cultures from hamster and monkey kidney cells. By 1965, the Japanese were
prepared to undertake large-scale Phase III trials of the lead candidate Nakayama strain vaccine. During the JE transmission season of that year, a collaborative group including the Taiwanese Department of Health, National University College of Medicine and Serum and Vaccine Research Laboratory; and the Japanese National Institute of Health and Nagoya University Medical College, conducted an Herculean study recruiting 110,166 children across four provinces of Taiwan6. One or two doses of Nakayama strain inactivated vaccine or tetanus toxoid placebo were administered prior to the peak transmission season and 62 cases of JE were detected among the volunteers in the following season. The efficacy in preventing JE was determined to be 80%. This initial vaccine was subsequently used in Taiwan’ and Japan8 to great effect.
In China, Dr Yu Yong Xin of the National Institute for Control of Pharmaceutical and Biological Products, Temple of Heaven, Beijing, developed the SA14 JE strain into an attenuated vaccine and the virus was adapted for growth in a canine cell line 9, and then tested by the Eckels and Trent at CDC to be stable and clean for human studies. 10 Xin then demonstrated the SA14-14-2 vaccine to be safe and immunogenic in a population of 1026 children and it was incorporated into the Chinese paediatric schedule of vaccinations in 1988. A case-control study conducted after an out break of JE in Sichuan Province (China), involving 56 confirmed cases of JE and 1299 controls, in which one dose previously administered was 80% and two doses approximately 97% effective. 11 After 100 million doses of the vaccine were used in China, Korean researchers conducted further studies to confirm safety and immunogenicity in their children ahead of licensure.12 The SA14-14-2 vaccine is used extensively in China, being manufactured by four agencies; in Korea by one private company and is under consideration by Health Departments in Thailand and Nepal.
By 1985, two groups of JE viruses were thought to exist. At this time, Dr. Charles Hoke and colleagues at the US Armed Forces Research Institute for Medical Sciences (AFRIMS) in Bangkok were preparing for a JE vaccine field efficacy trial in Thailand. On the advice of Konosuke Fukai of the Research Foundation for Microbial Diseases of Osaka University, Japan, Biken, a bivalent vaccine with the mouse brain-derived inactivated Beijing-1 JE vaccine strain, was used in the trial.
The landmark Thai study by AFRIMS, the Ministry of Public Health and Biken, forms the cornerstone of guidelines1 for the only internationally registered vaccine, the Biken Nakayama strain mouse brain-derived vaccine, JE – VAX %, distributed in Australia currently by CSL. The efficacy found by Hoke and colleagues in this study was 91% following two doses.13 Including the placebo group, 65,224 children were vaccinated; however, only 21,628 received the Nakayama strain vaccine alone with only one case of JE found in this group while 11 presented from the placebo group. Confidence intervals (95%) are high, ranging from efficacy of 70% to 97% as the foundation for efficacy of this vaccine is based on l3 cases of Thai children in an endemic area. A third group (n=22 080) received the bivalent vaccine and the efficacy figure quoted in Australia is actually that derived from the combination of the two JE vaccine recipient groups.
The Beijing-1 strain of JE was isolated in 1949. As 21 of the 162 cases of JE in Taiwan between 1986 and 1991 had been previously vaccinated with the Nakayama strain vaccine, the heterologous neutralising antibody response in children was reviewed to illustrate that the Beijing-1 strain vaccine was more immunogenic against wild strains circulating in Taiwan (CC-27 and CH-1392).15 As the Nakayama strain, JE was isolated in 1935, this may reflect a slow antigenic drift in the wild virus since, resulting in reduced immunogenicity of this strain. Today in Japan, Korea and Thailand, children are vaccinated with Beijing-1 strain mouse brain-derived vaccine.
The evidence for when to boost the Nakayama strain vaccine is based on less robust evidence. In the Mae Hong Son Province of Thailand, an area of low JE transmission, 199 children vaccinated were found to respond well (94% developed neutralising antibodies); however, their immunity decayed rapidly, so that more than half were susceptible after one year and a booster was given.16 Nevertheless, the Australian guidelines (Immunisation Guideline, 7th edition)< are based on the opportunity review of 39 US soldiers originally involved in the US licensure studies of the vaccine approximately three years earlier. 17 Only 17 soldiers could be adequately investigated to reach the conclusion that 16 (94%) had detectable antibodies persisting to three years. Australian Defence studies found 51-65% of soldiers retained detectable JE antibodies one year after vaccination.
DESIRE ATTRIBUTES IN FUTURE VACCINES
Though the JE virus is not known to mutate at a high rate, the immunogenicity of older vaccine strains has recently been called into question19. There are four genotypes (classes of genetic variation) of JE.20 These tend to follow loose geographic boundaries. All available vaccines are derived from genotype Ill strains. Notably, in January 2000, a second genotype (I), typically found in continental South-East Asia and the Korean Peninsula, entered the Australian region 21 to accompany the earlier incursion of a genotype (II) typical of Indonesia and Malaysia
The other problems with the existing inactivated mouse-brain derived vaccines are that they are quite reactogenic (cause reactions when given), require numerous doses, are expensive and ultimately are relying on mammalian neural tissue. 23 Therefore, an ideal prototype for second-generation JE vaccines would be grown in a pure cell line capable of up-scale for inexpensive production, have low reactogenicity and, after one dose, be immunogenic across all four genotypes or, at least, be comparable to the most recent vaccine strain (SA14-14-2).
THE VACCINES IN DEVELOPMENT
Japan
The Kanonji Institute (Osaka University) has begun moving their inactivated Beijing-1 strain from
mouse brain production lines to that of micro-carrierattached Vero cells. This vaccine will move into Phase III trials late in 2002. In competition in japan is the Chemo-Sero Therapeutic Research Institute (Kaketsuken) that has a similar Vero derived inactivated JE vaccine from the Beijing-1 strain approaching Phase II trials.
While the Vero cell line was originally from an African Monkey renal cell line, most of the commercially available Vero lines are considered highly purified of adventitious agents. It is also a favoured system for inexpensive up-scaling of production.
For final approval of these vaccines, the Japanese regulatory authorities are likely to accept serological outcomes (development of antibodies from the vaccine) rather than requiring the vaccine be tested and to protect in the face of wild virus. The issue then becomes which antibodies to accept as an outcome. Testing against the homologous vaccine strain (looking for antibodies to Beijing-1 grown in Vero cells) will probably provide a false positive to some degree when extrapolated to field efficacy of the vaccine, particularly against various genotypes.
Continental Asia
The Chinese SA14-14-2 strain live attenuated vaccine has been overwhelmingly effective as a public health intervention, reducing Japanese encephalitis in many areas of China. However, this vaccine has been produced in hamster kidney cell lines that have apparently met WHO/OMS standards of purity, though the national Vaccine and Serum Institute is moving the strain into Vero cells. The potential of this vaccine for the Developing World has not been missed with the WHO/OMS Western Pacific and South-East Asian Regional offices, in association with the Global Alliance for Vaccines and Immunisation (GAVI), producing a statement to accelerate international regulatory approval.
Similarly, the Glovax Corporation of Seoul, Korea has been collaborating with the Chengdu Institute
of Biological Products in China for validation of manufacture scale Vero cell vaccine production. The Republic of Korea has also managed to control epidemic JE using vaccination and is moving to intro duce this live attenuated vaccine into the childhood immunisation schedule to maintain control. Nevertheless, this vaccine is a live attenuated strain of a wild virus and is unlikely to be accepted for use in Australia while an inactivated and reasonably effective vaccine is licensed.
United States
The US Army at the Walter Reed Army Institute of Research (WRAIR) has been developing the SA14-14-2 strain as an inactivated vaccine. This program has completed initial human testing, including dose-ranging. The preliminary results are promising for a multi-dose schedule. Phase II trials are due for 2003; however, producing an inactivated vaccine in Vero cells will have low yield so that the vaccine will be expensive. While Barr Pharmaceuticals is collaborating in this project, the vaccine is primarily for the US military. The ADF is involved in negotiations to con duct further phase II trials: however, production of the vaccine has been delayed.
Europe
The chimeric virus approach to vaccine production has advanced most with JE. This technique inserts part of the JE genome into another virus used as a vector. The antigens expressed engender antibodies specific to JE. Recombinant vaccinia viruses were first synthesised in this manner 24, although now the 17D Yellow Fever vaccine strain is used as a backbone for the technology in a series of flavivirus vaccines25. The chimeric JE/17DYF vaccine successfully passed through Phase I studies recently. Despite the back bone, the vaccine does not stimulate immunity specific to Yellow Fever. An expanded Phase II trial, yet to be published in scientific literature, has confirmed the findings that the vaccine is safe and immunogenic (Acambis Press Release, January 2002). The
Australian Defence Force will take this vaccine into further phase II trials shortly.

FIGURE 2: Typical breeding site for Culex mosquitoes, in East Timor.
FURTHER HURDLES TO BE OVERCOME
With the incidence of JE, the total sample size for each of three groups of in excess of 20,000 Thai children in the Thai efficacy trial 13 barely demonstrated efficacy of the JE vaccine over tetanus toxoid in prevention of JE. For registration of future vaccines, “non-inferiority” Phase Ill efficacy trial would need to be larger again, proving preclusive in logistics and cost, particularly with a placebo group which would now be unethical to use.
At the last WHO/OMS Steering Committee on JE/Dengue vaccine development in Geneva in April
2002, Japanese manufacturers indicated local regulatory authorities might provide second-generation vaccines with the opportunity to complete phase III trials based on serological outcomes- antibodies being a surrogate of protection against the wild virus. This approach has been suggested for US regulatory approval7 though this is yet to be confirmed. With similar issues, the approach taken by the Australian Therapeutics Goods Administration is likely to be comparable to that of the US Food and Drug Administration.
CONCLUSION
In the next five years, several second-generation JE vaccines will be available. These will be less expensive, not produced in neurological tissue of rodents and immunogenic after fewer doses. The latter will particularly reduce the logistic cost of vaccine delivery Such vaccines will change the public health response to JE, allowing vaccine disease prevention to be more readily available to endemic populations. The ADF is well placed in the development of both the existing and second-generation JE vaccines to ensure personnel have the most efficient and effective protection against the “plague of the orient”.






