“Japanese encephalitis (JE), the “Plague of the Orient”, is the most important mosquito-borne viral encephalitis in Asia.”
INTRODUCTION
Japanese encephalitis is one of a number of arthropod borne viruses (arboviruses) known to cause human disease. It is a zoonoses of water birds and large mammals, such as pigs. It is usually transmitted by the bites of Culex spp. mosquitoes, mainly Cx. Tritaeniorhynchus and Cx. Gelidus. Human disease is associated with proximity to normal host populations, in particular pigs. Those at most risk of developing encephalitis are the young, due to not have been previously exposed, and the elderly, because of their reduced ability to ‘fight’ the disease. Other groups at risk include displaced persons such as refugees and long term expatriates living and working in rural settings.
JE is found throughout the Far East and SE Asia. It was until recently considered a disease of the Orient. Recently, however, there have been reports of JE cases in the Australasian region. Several vaccines are available. In Japan, use of Japanese Encephalitis vaccine has greatly reduced the transmission of JE.
DISCUSSION
Arboviruses
Arbovirus infection refers to those illnesses caused by arthropod borne viruses. These are commonly zoonoses maintained in a wild animal reservoir, often rodents and birds. Man is usually an incidental host and transmission occurs with proximity to infected animal reservoirs. There are some arboviruses, such as Dengue, that do not seem to rely on an animal reservoir. For others such as Japanese encephalitis, animal reservoirs are amplifying hosts for the illness. Arboviral infection can be present in a population without outward signs of infection as a result of a build up of immunity in the population. Epidemics occur when the immunity level drops, thus effecting the young and elderly most often, or those coming from non infected areas, such as peacekeeping forces.
There are four common clinical syndromes caused by arboviruses:
- Acute undifferentiated fever.
- Fever with a morbilliform rash.
- Fever with hepatitis and haemorrhagic features.
- Fever with encephalitis.
Japanese Encephalitis
Japanese encephalitis is caused by a flavivirus, transmitted by the bite of mosquitoes usually of the Culex spp. Initial symptoms include sudden onset with fever, headache and vomiting. For some, fits and/or disturbances in level of consciousness may follow. For every person with clinical JE there are likely to be from 25 to 1000 persons with subclinical infection. For those that develop encephalitis, there are three outcomes. For 25% of the cases, the outcome will be fatal. Of the survivors, 50% will develop permanent neurological damage. The rest will have a full recovery. Children are the main victims in epidemics as they have no immunity due to previous exposure. The same applies for large groups of people coming from areas where JE is not a risk. Thus, refugees and armies are susceptible during periods of epidemic transmission. For instance, the ratio of encephalitis cases to infection in American servicemen in Korea is 1:25. This compares to a ratio of 1:1000 children in Japan during times of epidemic transmission.
Distribution of JE
Wallace’s and Weber’s Lines
Alfred Russel Wallace, a nineteenth century naturalist, proposed a hypothetical boundary between the faunal regions of the Orient and Australasia. Wallace’ Line was a regional boundary or line extending from the Indian Ocean through the Lombok Strait (between the islands of Bali and Lombok), northward through the Makasar Strait (between Borneo and the Celebes), and eastward, south of Mindanao, into the Philippine Sea. This line presents an abrupt limit to the distribution of many fish, bird and mammal groups.
Lewis A. Japanese encephalitis: The plague of the Orient. Aust Mil Med 1999; 8(3), 3-6.
Capt. Tony Lewis is a Nursing Officer and is currently posted as the Health Intelligence Officer for the New Zealand Defence Force.

Figure 1: Crossing the Line
In 1902, a replacement was proposed. Weber’s Line extends from the Indian Ocean through the Timor Sea east of Timor, northward through the Molucca Sea (between the Celebes and the Moluccas), and into the Philippine Sea north of the Moluccas. Until recently Wallace’s Line was considered the usual eastward limit to the geographic distribution of JE. This may be changing, as there has now been JE spread to islands of the Torres Strait and reports of JE in Irian Jaya and Western Province of Papua New Guinea.6 Is it possible that JE could spread to NZ? Potential vectors certainly exist.
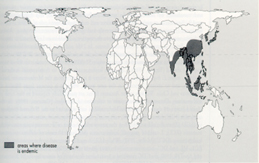
Figure 2: Distribution of Japanese Encephalitis
Vectors

Figure 3: Culex quinquefasciatus
Culex tritaeniorhynchus and Cx gelidus are the primary vectors of JE. These mosquitoes rarely enter houses. The adult mosquitoes feed throughout the night with peak biting activity at dawn and dusk, preferring wading birds and domestic animals. They feed on man during times of high vector population density. Larvae are found in a variety of sites including temporary ground pools, rice paddies, irrigation canals, bodies of polluted water and occasionally, artificial containers. Secondary vectors include Culex vishnui, Cx. Fuscocephala, Anopheles annularis and An. vagus.
Hosts
Birds and large mammals, especially pigs, are the favoured hosts. Man is an incidental host especially in rice growing areas or where there are pig farms. A recent outbreak of illness in Malaysia was thought to be JE as it was associated with areas of pig farming. This was eventually found to be a ‘Hendra’ type virus, not JE.
Risk
“The risk for acquiring JE is low for travellers. Only 11 cases have been reported in Americans since 1981, seven in military personnel or their dependents. In seven studies, JE attack rates among western military personnel in Asia have ranged from 0.05 to 2.1 per 10,000 per week with a median of 0.9. The low risk for acquiring infection and especially for developing symptomatic infection is explained by the following calculations. Typically, infection rates among vector mosquitoes are in the range of 5 per 1000. One estimate from India suggested that, during a transmission season, a child might receive 6000 bites from vector mosquitoes, of which only two might be infective. After infection, only one in 500 cases is symptomatic.”
Risk factors for disease relate to time, place and person:
There are two aspects to time; seasonality and host seeking activity of mosquito vectors. “Transmission is seasonal and occurs in the summer and autumn in the temperate regions of China, Japan, Korea and eastern areas of Russia. Elsewhere, seasonal patterns of disease are more extended or vary with the rainy season and irrigation practices.” 9 For transmission to man it also relies on mosquito densities which are effected by the seasonal variance.
The areas where JE is mostly transmitted are rural areas of the Far East and SE Asia where pigs are abundant and rice fields provide a suitable habitat for mosquito breeding. Urban areas can be at risk where they are in close proximity to pig farming.
Individuals are at risk when their activities take them into areas of high transmission, for instance, rice farmers. Age is also a factor due to lack of immunity or an inability to ‘fight’ the illness. Displaced persons into areas of JE risk, are particularly susceptible if no previous exposure and because of the likelihood of inadequate mosquito protection strategies.
For the visitor, the risk factors for acquiring JE infection are:
- Travel to endemic areas,
- Travel during transmission season,
- Extended period of travel or residence in the area,
- Outdoor activities, especially in the twilight period and evening,
- Poor protection against mosquito bites, and
- Lack of immunisation.
The risk to tourists travelling to endemic areas is quite low, less than 1:1,000,000. But the risk for visitors to rural endemic areas is increased during the transmission season to 1:5,000 per month. Despite this low risk, JE has occurred in a tourist to Bali after a short visit of only ten days.
PREVENTION
Primary means of protection against JE is avoidance of mosquito bites. Personal protection includes the use of DEET insect repellent on exposed skin, the use of mosquito nets and insect screens, permethrin impregnation of clothing and mosquito nets and vaccination. Community protection includes monitoring of JE serology in pigs, the control of mosquito breeding areas, and community education.
Where possible, persons in JE risk areas should use DEET based insect repellent on exposed skin surfaces. They should wear shirts with sleeves down and long trousers. Clothing and mosquito nets should be treated with permethrin to reduce mosquito biting. At night mosquito nets should be used and where possible rooms with window screens and air-conditioned. The idea is to stop place a barrier between the person and mosquitoes. These precautions also reduce the risk of other diseases such as malaria and dengue, which often occur in areas of JE activity.
Monitoring of JE serology in ‘sentinel’ pigs is one way of predicting outbreaks of the disease. Cases in the Torres Strait and one reported case in Cape York area have triggered concern over its spread to Australia in recent years.
It is very clear that control of mosquitoes is one area that can reduce the risk of JE to humans. This is only one area and it must be continually maintained. It requires community participation and government commitment. Longer-term strategies include vaccination of people and vaccination of host animals such as pigs.
All these strategies require education as often the disease is hidden from view. Such a strategy was employed in Malaysia during the suspected JE outbreak earlier this year.
The Vaccine
“Japanese Encephalitis Virus Vaccine Inactivated JE-VAX is a sterile, lyophilised vaccine for subcutaneous use, prepared by inoculating mice intracerebrally with Japanese encephalitis (JE) virus, “Nakayama-NIH” strain, manufactured by The Research Foundation for Microbial Diseases of Osaka University (“BIKEN “). Infected brains are harvested and homogenised in phosphate buffered saline, pH 8.0. The homogenate is centrifuged and the supernatant inactivated with formaldehyde, then processed to yield a partially purified, inactivated virus suspension. This is further purified by ultra-centrifugation through 40% w/v sucrose. The suspension is then lyophilised in final containers and sealed under dry nitrogen atmosphere. Thiomersal (a mercury derivative) is added as a preservative to a final concentration of 0.007% w/v. The diluent, Sterile Water for Injection, contains no preservative. Each 1.0 mL dose contains 11 mg of Tissue Culture Medium 199, 5 mg monosodium glutamate, 470 mcg of gelatin, less than 100 mcg of formaldehyde, less than 0.0007% v/v polysorbate 80 and less than 50 ng of mouse serum protein. No myelin basic protein can be detected at the detection threshold of the assay (<2 ng/mL). Prior to reconstitution, the vaccine is a white caked powder, and after reconstitution the vaccine is a colourless transparent liquid. The potency of JE-VAX is determined by immunising mice with either the test vaccine or the JE reference vaccine. Neutralising antibodies are measured in a plaque neutralisation assay performed on sera from the immunised mice. The potency of the test vaccine must be no less than that of the reference vaccine. “
The vaccine available in New Zealand is JE-VAX from CSL. It is available in New Zealand under Section 29 of the Medicines Act 1981 on a named patient basis.
It is considered to be at least 95% effective following its normal course of 0, 7 and 30 days. The vaccine can be given on days 0, 7 and 14 if there is insufficient time to vaccinate before departure. The vaccine may also be given as two doses on days 0 and 7, but this is considered to only confer up to 80% protection. In countries where JE is endemic, such as Japan, it is given as a two-dose course.
The current vaccine has a history of adverse reactions during the early 90s. It is thought that this may be due to a ‘bad’ batch and studies continue to assess the overall risk from the vaccine.13,14 There is an accepted rule, internationally, of allowing ten days from the last dose of the vaccine before departing overseas. This is to allow for medical care of individuals who may have a delayed adverse reaction requiring hospitalisation.
As a general rule of thumb, JE-Vax is given to those travellers going to areas of JE risk, during the risk period, and staying in rural environment for periods exceeding 30 days.
Treatment
Once symptomatic JE is present there is no specified treatment other than supportive care.
Conclusion
Japanese encephalitis is referred to as the “Plague of the Orient”. Its main victims are the young and the elderly. It is an arbovirus that is spread by the bite of mosquitoes, mainly of the Culex spp. The usual hosts for the disease are water birds and large mammals such as pigs and horses. Man is an incidental host, relative to his proximity to infected birds and domestic animals, the time of year and mosquito densities.
The risk of acquiring JE for the traveller is low. In areas of JE endemicity, it is likely that a substantial majority of the population will be serologically positive for JE. Those that become infected by JE are likely to be asymptomatic, however, of those that develop symptoms the fatality rate is 25%. Of those that recover, some 50% are left with neurological deficit.
There are vaccines for the prevention of JE. The one used in NZ is JE-Vax from CSL. It is available under Section 29 of the Medicines Act 1981. Concern has been raised about its safety due to adverse events in the early 90s. It is recommended for use for persons who will be staying in rural areas during times of JE transmission for periods greater than 30 days or for expatriates staying in JE endemic countries for over 6 months.
JE is spreading beyond its traditional region of the Orient. Concern has been expressed about its moving into Australia. Whether conditions are favourable for its spread to NZ could be debated, but it is important that one keeps an open mind and is always aware of the potential possibilities.






