Abstract
RSDL® (Reactive Skin Decontamination Lotion) Kit is approved for decontaminating chemical warfare agents and T-2 fungal toxins.
This work aimed to investigate the efficacy of RSDL or water irrigation for sulfuric acid dermal decontamination against untreated control in rabbit models.
Rabbits were randomly assigned to Groups 1 (no decontamination), 2 (water) or 3 (RSDL) and exposed to sulfuric acid (0.1 mL/3-4 cm2) for 30 seconds. Decontaminants remained on-site for 2 minutes.
Decontamination efficacy with RSDL and water were similar. Wound pH was higher and erythema persisted longer after decontamination versus control. Oedema developed by Day 3 and resolved by Day 15 in all groups. Necrosis started by Day 3, and all wounds had necrosis by Day 21. Wound areas following decontamination were significantly smaller than controls. Microscopically, all wounds in all groups had full-thickness epidermal and dermal necrosis.
In summary, RSDL and water were similarly effective in reducing wound size and increasing wound pH by Day 3 compared to controls. Histopathology data demonstrated similar tissue injury across all groups and showed no signs of wound healing after exposure. RSDL was a reliable decontamination method in the absence of immediate access to water.
Keywords: RSDL® (Reactive Skin Decontamination Lotion) Kit; sulfuric acid; dermal decontamination; wound healing; water irrigation
Introduction
Chemical skin burns (also known as caustic burns) differ from thermal burns. They can result from reduction, oxidation, corrosion or desecration of body tissue with/without thermal injury.1,2 Burn severity is determined by the chemical concentration, quantity, duration of contact and skin penetration of the chemical.2 The most important aspect of management is the removal of the agent from contact with the individual. Although chemical burns account for only 2%–4% of all burn injuries, they are associated with an increased duration of hospitalisation, particularly if the injury has occurred as the result of an assault.3 Approximately 5% of individuals with burns presenting to emergency departments require admission.4 According to the American Association of Poison Control Centers, there were >7000 exposures to acidic substances per year reported in 2016 and 2017.5,6
Sulfuric acid is an oily, colourless liquid used to manufacture products such as detergents, soaps, fertilisers, lead-based batteries and some common household cleaners.7 When splashed on skin (accidentally or intentionally), it produces dermal damage by dehydration, and thermal and chemical injury. Sulfuric acid is also a common acid used for physical assault attacks; the intent of an acid attack is not to kill but to cause permanent disfigurement.8,9 Many of these acid attacks take place in developing countries, with the highest incidence occurring in the West Indies and Bangladesh, where resources to deal with these scenarios may be limited.9 Criminal gangs may also use acid attacks as a form of punishment or intimidation.9 In these attacks, the head is often targeted and may result in severe deformation of the nose, destruction of ear cartilage or loss of eyelids or lips in survivors. Prompt management and decontamination are essential to reduce the impact of accidental or deliberate use of these caustic agents. The current standard of care for sulfuric acid injuries is to wash the wounded area with water for ≥15 minutes. While this method washes away any remaining acid, the immediate damage to the skin is difficult to prevent. Other decontaminants, such as Diphoterine® (Prevor Ltd.), a polyvalent solution, can be used for the management of acid skin injuries with some studies reporting a reduction in pain and wound healing time.10,11 However, evidence for the use of Diphoterine is not clearly established, as other investigators have questioned the evidence base and benefits of its use.12 Other options for immediate management after irrigation or in the absence of water include calcium gluconate gel and hexafluorine; however, the evidence to support their use is limited.13,14
The Reactive Skin Decontamination Lotion (RSDL) Kit was originally described by Fentabil et al.15 It has been shown to effectively remove or neutralise chemical warfare agents (CWAs) from the skin, including tabun, sarin, soman, cyclohexyl sarin, VR, VX, mustard gas and T-2 toxin, and has been well characterised in both in-vitro and in-vivo studies.15-19 RSDL is typically used as a carry-forward chemical countermeasure by various at-risk agencies (military and emergency responders) for timely use in the event of skin exposure to chemical warfare agents.
As mentioned previously, there is a trend of increasing cases of acid attacks in various parts of the world, which could lead to significant burn injury or even death.20 This has necessitated additional investigation of the RSDL Kit as an emergency intervention to counteract the consequences of the use of sulfuric acid outside military theatres and accidental spillage or contamination.21 Regarding emergency measures, immediate extensive irrigation with water and early excision of deep burns are universally accepted primary actions. However, further studies are needed to improve the early treatment of sulfuric acid burns.22,23 National Health Service guidelines in the United Kingdom recommend early intervention, removing clothing where possible, and continuously rinsing with clean water prior to paramedic arrival and support.24 However, water is not always readily available or transported in emergencies.
RSDL is alkaline (pH=10.6) and is expected to neutralise acids. A recent in-vitro study reported that the RSDL Kit effectively decontaminated sulfuric acid and hydrofluoric acid to 98.78% and 97.79%, respectively, on a non-porous, painted steel substrate.25 Therefore, in-vivo studies are warranted to assess the decontamination effect on live tissue injury and lesion healing process.
The current study aimed to investigate the in-vivo efficacy of the RSDL and water irrigation for dermal decontamination of sulfuric acid in a rabbit model compared with untreated control. The rabbit model was chosen based on Organization for Economic Cooperation and Development (OECD) recommendation and data obtained from the pilot study described above.26
Material and methods
Chemicals
The RSDL (500 mL; lot number 23000933) and applicator sponge were obtained from Emergent BioSolutions Canada Inc. (Winnipeg, Manitoba, Canada). Water used in these experiments was purified using a Barnstead International Inc. (Dubuque, IA, USA) Nanopure Diamond UV Series 1191 ultrapure water system, which purifies water to a resistance of 18 MΩ cm. Water for injection was purchased from MWI (Boise, ID, USA). Sulfuric acid (CAS: 7664-93-9) was purchased from Sigma-Aldrich (lot number SHBF5690V; 96.3% purity).
Animals
A total of 15 (12 on study with three extra/replacements) male New Zealand white rabbits, which weighed 3.1–3.6 kg and were ≥3 months old at the time of arrival, were received from Covance Research Products (Denver, PA, USA). The study protocol was approved by MRIGlobal Institutional Animal Care and Use Committee in compliance with US guidelines. General procedures for animal care and housing were in accordance with the ‘Guide for the Care and Use of Laboratory Animals’. 27
Prior to inclusion into the study, animals were inspected for health status and quarantined in the conventional animal facility for ≥7 days. Each rabbit was tattooed on one ear with a unique alphanumerical designation by the vendor. All animals included in the study were in good health and free from any signs of clinical disease prior to inclusion.
All rabbits were provided with certified feed (Harlan Rabbit Diet) and fresh tap water (Kansas City Municipality) ad libitum. The rabbits were singly housed in conventional rabbit caging and environmentally controlled rooms with at least 10 air changes per hour. The rooms were maintained at a temperature of 61°–72°F (16.2°–22.3°C) and a relative humidity of 50% ± 20% with a 12-hour light/dark cycle/day. Room temperature, humidity and light cycles were monitored 24 hours/day throughout this study.
As the corrosive challenge agent was expected to cause pain by inducing caustic wounds, sustained-release (SR) buprenorphine (ZooPharm, Laramie, WY, USA) was given via intramuscular (IM) injection prior to sulfuric acid application and continued throughout the study to ameliorate pain and animal suffering. Analgesic dosages were tailored to each animal based on weight at the discretion of the attending veterinarian. At the conclusion of the study, rabbits were anaesthetised with ketamine (≤60 mg/kg; VetOne, Boise, ID, USA)/xylazine (≤5 mg/kg; VetOne, Boise, ID, USA) by IM or subcutaneous injection prior to euthanasia with Euthasol (≥1.0 mL/4.5 kg; VetOne, Boise, ID, USA) administered by cardiac puncture or intravenously (IV).
Study design
Animals were randomly assigned, based on body weight, to one of three treatment groups with four animals in each group. Group 1 rabbits were not decontaminated post-sulfuric acid exposure. These rabbits remained in the chemical fume hood for ≥15 minutes post-sulfuric acid exposure and were then returned to their home cage. Group 2 and Group 3 rabbits were decontaminated via water irrigation or with the RSDL Kit. Each animal was then returned to its single cage for periodic monitoring and sample collection.
Animal preparation
Prior to Study Day 0, rabbits had an area of approximately 124 cm2 on the dorsal surface of their back (across the dorsal midline) close-clipped. Six sites (three opposite each other) across the dorsal midline were designated for sulfuric acid application, and subsequent biopsies were identified using indelible ink. On Day 1, sulfuric acid (0.1 mL/3–4 cm2) was applied to the anaesthetised rabbits for 30 seconds at all six designated sites.
Skin decontamination
The RSDL Kit application to the corrosive contaminated dermal sites occurred 30 seconds post-sulfuric acid exposure. The 10´10 cm sponge component of the RSDL Kit was cut into four approximately equal pieces, each placed in a clean weigh boat, and 10 mL RSDL was applied to each piece using a positive displacement pipette. The RSDL-soaked sponge (Kit) was used to rub each site firmly for 10 seconds to lift off the maximum amount of corrosive. Afterwards, the lotion remained on the site for 2 minutes before being firmly wiped off with clean, wet gauze using sterile water.
Water irrigation occurred 30 seconds post-sulfuric acid dermal exposure. A 10 mL syringe filled with 10 mL sterile water was used to rinse each corrosive site for 10 seconds at approximately 1 mL/second. The sterile water was applied to rinse the exposure site and run off the back of the rabbit. The water remained on the site for 2 minutes before removing the excess using clean, wet gauze.
Clinical observations
Routine clinical observations were performed daily throughout the quarantine and acclimatisation period. Clinical observations were performed just prior to corrosive chemical application and twice daily post-exposure. Abnormal clinical observations outside specified time points were also recorded on appropriate data capture forms.
Wound observations and measurements
The pH of each wound was tested using litmus paper (GE Healthcare-Whatman, Chicago, IL, USA). For Group 1 (animals not decontaminated post-sulfuric acid exposure), pH was measured immediately after exposure. For Groups 2 and 3 (wounds decontaminated with water or the RSDL Kit), pH was measured immediately post-decontamination, again after the wounds had been wiped with gauze, and on Day 3 after dampening the wounds with sterile water.
Modified Draize Scoring28 was recorded prior to sulfuric acid exposure and on Days 3, 15 and 21 and included necrosis, erythema and oedema. Scar description was recorded and photographed to include form, size, discolouration and density.
The wound area at each dose site was measured directly after sulfuric acid application and again on Days 3, 15 and 21. Wound length was defined as the most dorsal point to the most ventral point of each wound based on the dorsal-ventral axis of the animal. Wound width was defined as the most cranial point to the most caudal point of each wound based on the cranial-caudal axis of the animal. The length and width were multiplied to calculate the area. Photographs of shaved dorsal sites were captured for all animals prior to exposure and on Days 3, 15 and 21.
Wound biopsy
Biopsies were performed on Days 3, 15 and 21. Two of the six predesignated sites opposite each other on each animal were biopsied each day, starting with the most cranial sites on Day 3 and ending with the most caudal sites on Day 21. After premedication with SR buprenorphine and anaesthesia, biopsies were performed on Days 3 and 15. The animals were re-shaved if necessary, and a sterile field was prepared by first wiping the area with sterile saline followed by a chlorhexidine scrub (2/rabbit; eight wounds per group). A surgical drape was placed to isolate the biopsy site. Approximately 0.5–1.0 cm away from the wound, healthy tissue was gently grasped using atraumatic forceps. The healthy skin and wound were excised using dissecting Mayo scissors with a 0.5–1 cm margin of healthy tissue. Skin biopsies were placed into a pre-labelled cassette and 10% formalin (10:1 formalin-to-tissue ratio). If necessary, the wounds were closed using sutures, skin stapes and skin glue. The animals were then allowed to recover from anaesthesia. The biopsy wound sites were monitored for the remainder of the study for signs of infection.
On Day 21, biopsies were performed using the same procedures as above. Once the remaining wounds were harvested, the rabbit was euthanised via IV or cardiac puncture with euthanasia solution.
Histopathology
Collected skin samples were fixed in formalin for ≥72 hours and then shipped at ambient temperature to HSRL, Inc. (Mount Jackson, VA, USA) for processing, followed by slide preparation stained with hematoxylin and eosin (H&E) for conventional light microscopy by Singer & Associates Toxpath Consulting Solutions, LLC (Ponte Vedra, FL, USA). One slide was prepared from each dose site.
A certified veterinary pathologist, blinded to the decontamination methods and treatments, reviewed the H&E-stained slides and provided subjective wound site healing and re-epithelialisation assessments using the scoring criteria of re-epithelialisation, abnormal epidermal cells, basement membrane, hair follicles, vascular proliferation, haemorrhage, dermal necrosis changes and dermal inflammation.
Statistical analyses
Wound area was analysed using pairwise marginal means estimates, and modified Draize scores were analysed using Type III test for fixed effects analysis of variance performed using SAS Version 9.4 (SAS Institute Inc., Cary, North Carolina) to discern the efficacy of the RSDL Kit and water decontamination procedures versus no decontamination for the treatment of corrosive dermal wounds.
Results
Wound pH
The wound pH means in treatment groups are shown in Table 1.
Modified Draize scoring
All animals exposed to sulfuric acid developed erythema within 3 days post-exposure (Table 3). Erythema persisted until Day 15 for all groups, with Group 1 (no decontamination) having the highest scores (1–3) compared with Groups 2 and 3 (water and RSDL Kit, respectively, range 0–2). Groups 2 and 3 had significantly lower erythema scores on Study Day 15 (p< 0.05). By Day 21, only Groups 2 and 3 had erythema scores ranging between 0–1. Scores for Groups 2 and 3 were significantly lower than for Group 1 (Table 2).
Table 1. Wound pH
| Group ID | Decontamination | Post-sulfuric acid application | Post-gauze wipe | Day 3 post-exposure | |||
|---|---|---|---|---|---|---|---|
| Mean pH | Std Dev | Mean pH | Std Dev | Mean pH | Std Dev | ||
| 1 | No decontamination | 0.00 | 0.00 | NA | NA | 3.42 | 0.91 |
| Post-decontamination | Post-gauze wipe | Day 3 post-exposure | |||||
| 2 | Water | 1.00 | 0.00 | 1.00 | 0.00 | 5.00 | 0 |
| 3 | RSDL Kit | 1.67 | 0.30 | 1.21 | 0.21 | 4.54 | 0.55 |
NA, not applicable; RSDL, Reactive Skin Decontamination Lotion Kit; Std Dev, standard deviation.
Table 2. Sulfuric acid dermal exposure erythema, oedema and necrosis scores (Modified Draize scores)
| Erythema scores* | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day 0 | Day 3 | Day 15 | Day 21 | |||||||||||||||||
| N | 0 | 1 | 2 | 3 | N | 0 | 1 | 2 | 3 | N | 0 | 1 | 2 | 3 | N | 0 | 1 | 2 | 3 | ||
| 1 | None | 24 | 24 | 0 | 0 | 0 | 24 | 0 | 22 | 1 | 1 | 12 | 0 | 6 | 2 | 4 | 6 | 6 | 0 | 0 | 0 |
| 2 | Water | 24 | 24 | 0 | 0 | 0 | 24 | 0 | 21 | 0 | 3 | 16 | 0 | 12 | 4 | 0 | 8 | 6 | 2 | 0 | 0 |
| 3 | RSDL Kit | 24 | 24 | 0 | 0 | 0 | 24 | 0 | 12 | 12 | 0 | 16 | 1 | 14 | 1 | 0 | 8 | 5 | 3 | 0 | 0 |
| Oedema scores† | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day 0 | Day 3 | Day 15 | Day 21 | |||||||||||||||||
| N | 0 | 1 | 2 | 3 | N | 0 | 1 | 2 | 3 | N | 0 | 1 | 2 | 3 | N | 0 | 1 | 2 | 3 | ||
| 1 | None | 24 | 24 | 0 | 0 | 0 | 24 | 0 | 0 | 24 | 0 | 12 | 12 | 0 | 0 | 0 | 6 | 6 | 0 | 0 | 0 |
| 2 | Water | 24 | 24 | 0 | 0 | 0 | 24 | 2 | 4 | 18 | 0 | 16 | 16 | 0 | 0 | 0 | 8 | 8 | 0 | 0 | 0 |
| 3 | RSDL Kit | 24 | 24 | 0 | 0 | 0 | 24 | 0 | 0 | 24 | 0 | 16 | 16 | 0 | 0 | 0 | 8 | 8 | 0 | 0 | 0 |
| Necrosis scores‡ | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day 0 | Day 3 | Day 15 | Day 21 | |||||||||||||||||||||
| N | 0 | 1 | 2 | 3 | 4 | N | 0 | 1 | 2 | 3 | 4 | N | 0 | 1 | 2 | 3 | 4 | N | 0 | 1 | 2 | 3 | 4 | ||
| 1 | None | 24 | 24 | 0 | 0 | 0 | 0 | 24 | 20 | 2 | 1 | 0 | 1 | 12 | 0 | 0 | 0 | 4 | 8 | 6 | 0 | 0 | 0 | 0 | 6 |
| 2 | Water | 24 | 24 | 0 | 0 | 0 | 0 | 24 | 24 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 5 | 11 | 8 | 0 | 0 | 0 | 0 | 8 |
| 3 | RSDL Kit | 24 | 24 | 0 | 0 | 0 | 0 | 24 | 21 | 3 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 1 | 15 | 8 | 0 | 0 | 0 | 0 | 8 |
N = Number of evaluated sites with sulfuric acid application
*Erythema scoring: 0 = Unchanged from an uncontaminated area of skin; 1 = Mild erythema; 2 = Moderate erythema; 3 = Severe erythema.28
†Oedema scoring: 0 = Unchanged from an uncontaminated area of skin; 1 = Mild oedema; 2 = Moderate oedema; 3 = Severe oedema.28
‡Necrosis scoring: 0 = Unchanged from an uncontaminated area of skin; 1 = Focal necrosis (focal area[s] of tissue [is/are] necrotic); 2 = Mild Necrosis (25–50% of the tissue is necrotic); 3 = Moderate necrosis (50–75% of the tissue is necrotic); 4 = Severe necrosis (75–100% of the tissue is necrotic).28
RSDL Kit, Reactive Skin Decontamination Lotion Kit.
Oedema developed within 3 days of exposure in all groups. All wounds in Group 1 scored a 2, wounds in Group 2 had scores of 0–2, and all wounds in Group 3 had a score of 2. Oedema had resolved by Day 15 (Table 3). On Day 3, Oedema was significantly lower in Groups 2 and Group 3 compared to Group 1 (p< 0.01).
Necrosis started to develop in the wounds of Groups 1 and 3 by Day 3. The scores ranged from 0–4 for Group 1 and 0–1 for Group 3. Group 2 did not have any necrosis on Day 3. By Day 15, all wounds in all groups had necrosis (score range 3–4). By Day 21, all wounds in all groups were scored at 4 (Table 3). There were no statistically significant differences between the groups in the context of necrosis.
Wound area
The results of would area measurements over time are presented in Figure 1. Animals in all groups developed wounds within 3 days of sulfuric acid exposure. Decontamination decreased the level of tissue injury and led to consistently smaller wound sizes in Groups 2 (water) and 3 (RSDL Kit) compared with Group 1 (no decontamination; P≤0.0001).
Moreover, in Group 3, progressive wound area reduction was demonstrated with time, unlike those without decontamination (Table 3). Post-hoc least squares means tests were performed to determine significant differences between marginal means at each time point for each group level. Pairwise comparisons show estimated differences in marginal group means and adjusted P values (Table 3). For the decontamination of sulfuric acid from rabbit skin, our data show that RSDL Kit performs similarly to water.
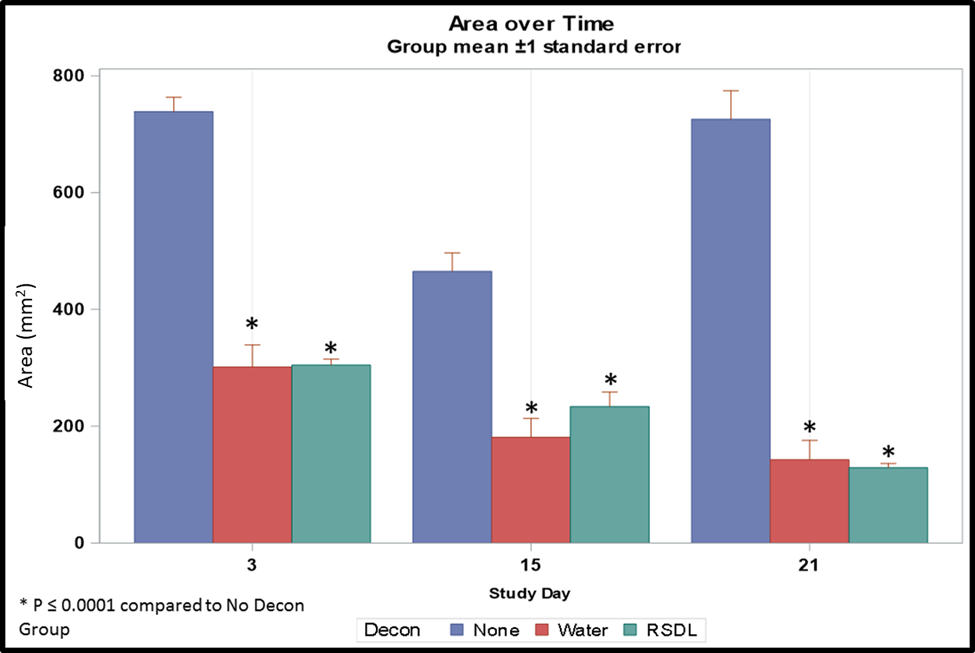
Figure 1. Dermal wound area post-sulfuric acid exposure with no decontamination, water decontamination and decontamination using the RSDL Kit post-exposure. Decon, decontamination.
Table 3. Pairwise marginal means estimates—area
| Group | Timepoint contrast (days) | Difference in wound size | t value | Adjusted P value | ||
|---|---|---|---|---|---|---|
| 1 | None | 3 | 15 | 275.64 | 11.03 | <0.0001 |
| 3 | 21 | 34.4522 | 1.05 | 0.5498 | ||
| 15 | 21 | −241.19 | −7.07 | <0.0001 | ||
| 2 | Water | 3 | 15 | 76.5659 | 3.50 | 0.0025 |
| 3 | 21 | 170.09 | 5.94 | <0.0001 | ||
| 15 | 21 | 93.5209 | 3.17 | 0.0068 | ||
| 3 | RSDL Kit | 3 | 15 | 121.91 | 5.57 | <0.0001 |
| 3 | 21 | 161.62 | 5.64 | <0.0001 | ||
| 15 | 21 | 39.7122 | 1.34 | 0.3768 | ||
Histopathology
Due to opening wounds and insufficient healthy skin to close biopsy sites, one animal in Group 1 (no decontamination) whose wounds opened up after the Day 3 biopsies were removed from the study by Day 7. All remaining wounds for this animal were harvested on Day 7.
Microscopically, all wounds had full-thickness epidermal and full-thickness dermal necrosis, regardless of decontamination procedures. Necrosis continued into the hypodermis, generally with some degree of skeletal muscle necrosis. Based on the necrosis observed in all wounds, there was no evidence of wound healing in any group, regardless of biopsy day. Representative photomicrographs of the dose sites throughout the study are included in Figure 2.
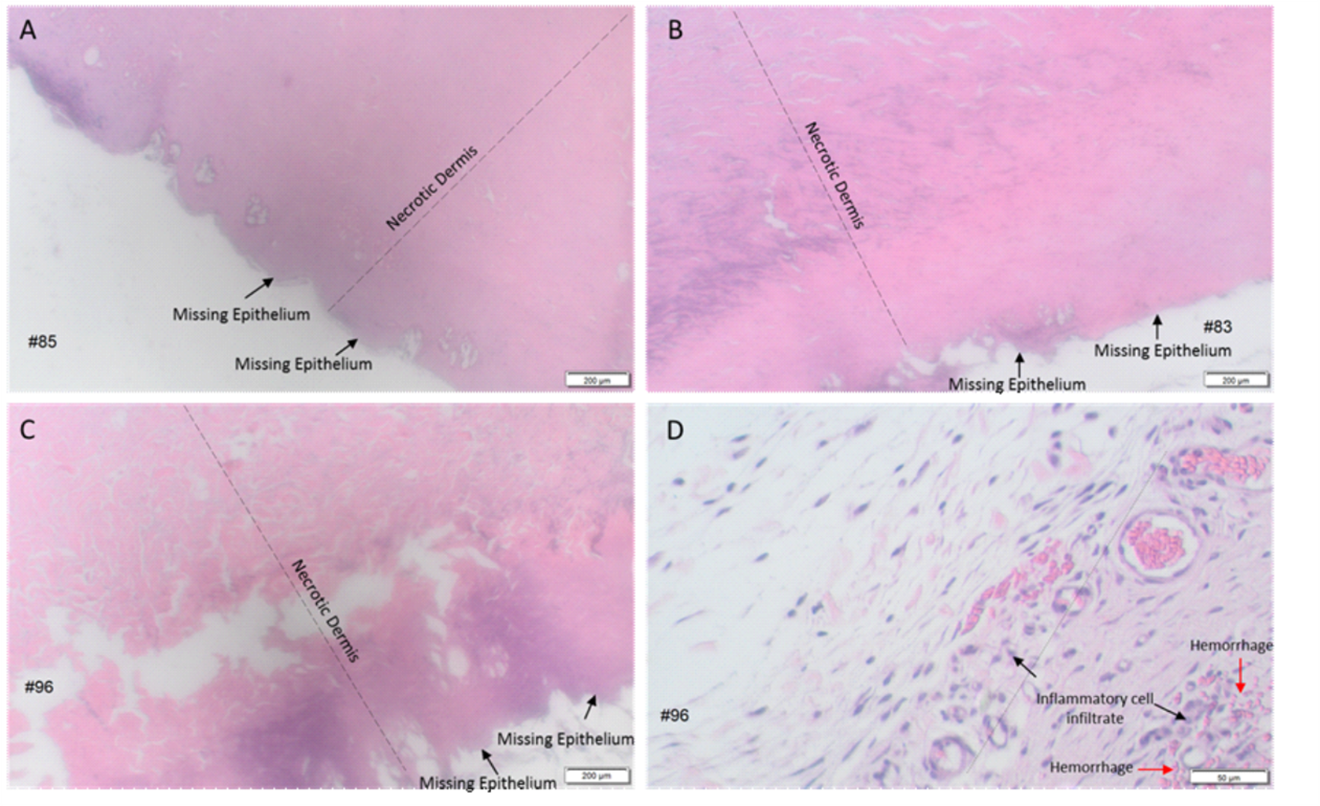
Figure 2. Representative photomicrographs of wounds exposed to sulfuric acid with no decontamination, water and the RSDL Kit. A–C) 2X images, all wounds had missing epithelium and necrosis from epidermis to hypodermis (not shown) A) wound harvested on Day 7 from rabbit that received no decontamination post-sulfuric acid exposure B) wound harvested on Day 15 from rabbit that received water irrigation post-sulfuric acid exposure C) wound harvested on Day 21 from rabbit that received decontamination using the RSDL Kit post-sulfuric acid exposure. D) 10X image, hypodermis-dermal junction with inflammatory cell infiltrate and haemorrhage in wound harvested on Day 21 from rabbit that received the RSDL Kit decontamination post-sulfuric acid exposure.
Discussion and conclusions
This study examined the effect of the RSDL Kit on wound healing compared with no intervention or water irrigation for dermal decontamination of sulfuric acid exposure in the rabbit model. This model accurately predicts severe human skin injury but may have some limitations in distinguishing mild and moderate changes.29
Overall results of this study demonstrated that the effectiveness of the RSDL Kit was equivalent to water irrigation treatment with respect to wound size and pH, while the histopathology data demonstrated that all wounds across all groups were similar due to initial strong acid corrosive injury. Though the rabbit model may not fully reflect the human scenario, RSDL appears to have reasonable applicability in managing caustic skin damage without immediate access to water.
Both water (Group 2) and the RSDL Kit (Group 3) were equally effective in managing sulfuric acid skin exposure. Wound areas decontaminated with water or the RSDL Kit were significantly smaller than wound sites without decontamination (Group 1). The wounds in Group 1 continued to worsen during the in-life observations of this study due to the lack of decontamination procedures and did not demonstrate signs of healing or stabilisation of the injury. The pH of wounds in Groups 2 and 3 was higher than in Group 1. Erythema persisted to Day 21 in Groups 2 and 3 compared with Group 1, which had no erythema on Day 21. The absence of erythema together with persistent necrosis/wound area could be evidence of no wound healing, as has been shown for laser burn in nontreated animals.30 Oedema developed by Day 3 in all groups and resolved by Day 15. Necrosis started in Group 1 and in Group 3 (to a lesser degree) by Day 3, but all wounds had necrosis by Day 15 and 21. Microscopically, all wounds in all three groups had full-thickness epidermal necrosis and full-thickness dermal necrosis. Necrosis continued into the hypodermis, with some degree of skeletal muscle necrosis in all wound sites.
While decontamination with the RSDL Kit (Group 3) and water irrigation (Group 2) effectively reduced wound size and increased wound pH by Day 3, the histopathology data demonstrated that all wounds across all groups were equally damaged after the strong corrosive acid exposure. In this study, the RSDL Kit was not inferior to standard emergency treatment with water irrigation for decontaminating the burns caused by sulfuric acid. In the absence of immediate access to water, the results of the current study support the use of RSDL Kits as a reliable decontamination method.
This work was performed with some limitations to address specific medical gaps related to the timely and effective removal of acid from the skin at the first point of care and where immediate access to water is not available and not with the expectation of modification of current medical policies. The fully (96.3 %) concentrated sulfuric acid used in this study may have been too caustic to demonstrate any improvement in wound healing with either form of skin decontamination, but concentrations of up to 98% are found in many commercial products, supporting the use of the full concentration in this study.2,31 Further studies to evaluate the efficacy of the RSDL Kit, water irrigation, and other methods of decontamination on wound healing after exposure to sulfuric acid are needed to develop guidance for the management of accidental or malicious exposure to sulfuric acid and other caustic compounds.
Corresponding Author: Vladimir Savransky, savranskyv@ebsi.com vladimir.savransky@gmail.com
Authors: V Savransky1, P Anantharam2, L Cochrane3, J Barry1, J Mikler4
Author Affiliations:
1 Emergent BioSolutions Inc – Preclinical R&D
2 MRIGlobal
3 Emergent Countermeasures International Ltd.
4 Defense Research and Development Canada Suffield Research Centre


