Introduction
The current coronavirus (COVID-19) pandemic is a stark reminder of the burden of disease for non-battle injury (DNBI) on militaries worldwide. Operational capability impacts both through direct morbidity and mortality of DNBI and the readiness expected of military healthcare workers to either augment saturated civilian healthcare services or substitute service provision during the breakdown of services, such as in humanitarian emergencies. This often includes the provision of vaccinations against vaccine-preventable diseases (VPD) in remote or austere environments. For example, at the height of the 1918–19 influenza pandemic, an estimated 20–40% of United States (US) military personnel suffered influenza and pneumonia, causing more deaths than from enemy action during World War I.1,2 As this pandemic extended to Australia, about 40% of the population became ill and approximately 15 000 died.3
Contemporary outbreaks of Ebola, influenza A and B, hepatitis E and other infectious diseases continue to be documented in the militaries of many nations and can threaten to overrun the limited capacity of deployed healthcare facilities.4-7 These events have led to a significant logistical burden, with resources being diverted from combat service support to the provision of healthcare and casualty transport for DNBI morbidity and mortalities. Vaccinations are a key force protection measure to ensure enduring operational capability. They are a basic and critical health intervention for vulnerable displaced populations in humanitarian emergencies.8
Accounting for variability in vaccines, diseases and their emerging variants, vaccinations are generally highly effective in protecting these populations from contracting or suffering severe illness from VPD. Several public health and logistics challenges are unique to a deployed and often austere military environment. These include the need for cold or ultra-cold storage and shipment logistics chains and balancing consumption and scheduling to avoid wasting limited vaccinations. This study aims to detail the experience and highlight some challenges in COVID-19 vaccination provision at a deployed healthcare facility in an austere environment.
Materials and methods
This is a descriptive study of the COVID-19 vaccination program from March to October 2021 of a deployed US Army-led multinational Role 3 healthcare facility in a field hospital in Iraq during Operation Inherent Resolve. The inception of the vaccination program, including key personnel, roles and training requirements, is described. The number, types and rates of vaccinations and adverse events are reported.
The inclusion criteria consisted of all US, coalition partners and local nationals that were vaccinated with either Pfizer-BioNTech (BNT162b2 [Pfizer]), Moderna (mRNA-1273 [Moderna]) or Janssen (Johnson & Johnson; Ad.26.COV2.S [J&J]) vaccines. These were the only vaccines authorised for distribution at and by the reporting clinic. A satellite vaccine clinic whereby the Role 3 staff visited the local potable water factory to logistically accommodate additional base personnel was also established. The exclusion criteria consisted of any COVID-19 vaccines not mentioned above. In addition, COVID-19 vaccines delivered at outlying military facilities (not in the Role 3 catchment area) were excluded.
The vaccine drive set-up required four primary coordinating personnel in addition to those executing the actual administration of the vaccine. Our coordinating team consisted of an overall coordinator, lead clinical coordinator, lead operations coordinator and pharmacist. Table 1 details the roles and responsibilities of these key leaders. Staff administering vaccinations completed a minimum of four online Centres for Disease Control and Prevention (CDC) training modules, including ‘You Call The Shots’ and specific COVID-19 vaccine training modules dedicated to each vaccine type administered.9,10
Table 1 – COVID-19 Vaccination – Key personnel
| DUTY TITLE | DUTY DESCRIPTION |
|---|---|
| Overall Coordinator | Responsible for the communication between all parties to ensure a unified message and streamlined information flow. |
| Should be well-versed in both military and medical terminology to meet all operational and clinical demands. | |
| Maintained responsibility for de-conflicting numerous challenges and for selecting time and place for the vaccine drive. Also constructed the medical message for base-wide distribution. | |
| Lead Clinical Coordinator | Maintained responsibility for conducting the vaccine execution. |
| Maintained responsibility for conducting the vaccine execution. | |
| Lead Operational Coordinator | Maintained responsibility for conducting liaison operations outside the Role 3 to inform other base commands and recruit for maximum participation. |
| Ensured the operational feasibility of the plan for the military mindset. | |
| Pharmacist | Pharmacist Responsible for coordinating proper storage and availability of vaccines. |
| Helped make informed decisions regarding the timing of vaccine removal and time of expiration. | |
| Maintained situational awareness of shelf-life extensions and other relevant changing guidelines |
Figure 1 – Vaccination clinic patient flow
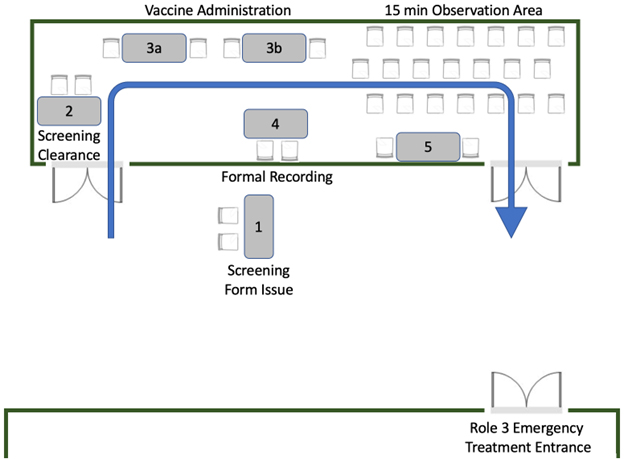
Once the vaccine was delivered, patients remained on location at the Role 3 facility for a minimum of 15 minutes to ensure no immediate adverse reactions. A schematic of the flow of the vaccination program is shown in Figure 1. Additionally, as the vaccine drive was conducted on the Role 3 premises, emergency medical personnel were always readily available. Of note, in the case of the offsite water factory mission, only experienced personnel with emergency medication knowledge administered vaccines. In addition, the water factory facility also provided intrinsic medical assets as redundancy. Treatment protocols included standard anaphylaxis medications (Diphenhydramine, Methylprednisolone, Epinephrine injection).
In addition, some of the unique challenges of COVID-19 vaccinations in this deployed environment are discussed. This includes issues surrounding cold chain logistics and batch dose vials, the public health campaign, vaccine hesitancy and non-compliance with the vaccination policy.
Results
During the study period, 755 COVID-19 vaccine doses were administered at the Role 3 healthcare facility; 236 vaccine doses were either the Pfizer or Moderna vaccine and 519 were single doses of the J&J vaccine. Of these administered doses, 40 were given as booster vaccines (10 Pfizer and 30 Moderna). Due to difficulty with temperature-controlled storage and shipment, the facility received and administered J&J or Moderna COVID-19 vaccine almost exclusively, with the rare ability to secure and administer Pfizer. Thus, the Pfizer vaccine represents only a minority of the doses administered. The US military personnel represented 361 (47.8%) of the vaccine doses administered. In addition, approximately 30 vaccine doses were administered to employees of a local company contracted to provide potable water services for camp personnel, ensuring minimal impact on our operations. We recorded two discreet incidents (0.26%) of patients with presyncope type episodes after vaccination. There were no patient reviews for common symptoms such as myalgia, headache or malaise. There were no cases of anaphylaxis or any other major adverse event.
Discussion
As long as there has been warfare, DNBI has significantly impacted the health of military personnel and the operational capabilities and readiness of armies. Historically, it has greatly outnumbered the impact of combat-related traumatic injuries. For example, the Spanish Influenza pandemic in 1918 alone likely killed more military personnel than died in combat during World War I.2 Similarly, a typhoid fever epidemic accounted for more US service member deaths than combat during the Spanish–American War. Despite modern advances and preventative strategies, DNBI remains a major concern, accounting for 75% of hospitalisations in the early stages of Operation Iraqi Freedom.11
Deployed militaries were not spared from the threat of the COVID-19 pandemic. The characteristics of combat deployments with the inability to socially distance, and shared dining and ablution facilities may adversely affect the transmission rate and subsequently place additional burdens on limited medical resources.2 While the relatively young age and lack of severe comorbidities of service members appear to be protective, medical systems in the deployed setting—focused on rapid management of combat casualties—often lack redundancy in equipment, medications and staffing to address a large influx of ill patients and may also lack advance therapies for the critically ill.12 Likewise, the safe transport of COVID-19 patients to facilities with adequate resources poses major challenges, including maintaining infection control precautions, ensuring adequate oxygenation during flight, and decontamination of evacuation platforms. These issues have been used to justify a growing call for COVID-19 vaccination mandates in many militaries worldwide.13
Routine vaccinations for military personnel are minimised during operations in austere environments to avoid storage and shipping difficulties. Preventative healthcare and pre-deployment preparations ensure appropriate vaccinations are administered before entering a combat theatre. Nevertheless, a capacity for critical multidose immunisations that are often started but not completed before mobilisation is maintained. This vaccination capability proved to be the backbone for our COVID-19 vaccination program to be developed and expanded. Exhaustive healthcare planning still cannot entirely anticipate all medical threats to deployed military personnel and flexibility is paramount. The COVID-19 pandemic highlights the continued need to be able to rapidly deploy immunisations in response to future epidemics or even in response to bioweapons.14
Program implementation should emphasise human factors, including staff training, contraindication screening, recipient education and attention to patient identification and documentation. Established and effective pathways enhance adaptability in addressing the current pandemic and future unanticipated medical challenges.
Challenge: Cold chain logistics and batch dose vials
As expected, the cold chain management presented numerous problems with the logistical execution of the vaccine delivery. Chief among them was possession of a freezer with the capability of storing vaccines at -80ºC to -60ºC (Pfizer) or -50ºC to -15ºC (Moderna).15,16 The Role 3 laboratory freezer could accommodate the Moderna temperatures. Once thawed, the vaccines had approximately 30 days of shelf life.15 Due to the logistical ease, including the requirement for only one dose, the US military temporarily opted for J&J vaccines. The single-shot option was also more agreeable to many patients. In our case, an adjacent Department of State health facility possessed the freezer storage capability. Without this collaboration, we would have been relegated to the expiration dates for thawed vaccines, which would further constrain our planning options. Though there were no breaches to cold chain requirements in our facility, there has been a reported case of a brief (5 hour) breach to cold storage requirements in a hospital in Spain, which had no consequence on the integrity of Moderna vaccines.17 Should a breach in the cold chain requirements have occurred, a cold chain breach protocol, such as the Australian National vaccine storage guidelines, ‘Strive for 5’ (3rd ed.) would have been employed.18 Such protocol would be adapted for a deployed context but generally involves isolation of the vaccines, transfer back to refrigeration requirements and labelled as ‘Do not use’ until clarification from the relevant health department or authority. Control mechanisms to adhere to manufacturers’ recommendations are paramount, but further information relating to the packaging, handling and storage of vaccines is particularly relevant in limited-resource deployed environments.
An explicit directive forbid wasted doses, further complicating the delivery. The companies transported multidose vials of Moderna in vials of 10 or 15 doses, Pfizer in vials of five doses and J&J in five doses.15 Coordinating among multiple units to arrive en masse requires a diligent networking lead operations coordinator. We recommend setting expectations on arrival that up to 5 (or 10) patients may be rescheduled if the correct multiple is not available.
Prioritising high-risk personnel, such as healthcare workers and those in liaison roles, also required delicate messaging. Once the first dose of a vial is utilised, a time restriction for administering all doses is commenced. The expirations from the time of seal removal are as follows: Moderna: 12 hours, Pfizer: 6 hours, and J&J: 6 hours.15,16 Given these relatively stringent timeframes, we recommend having the requisite number of vaccine recipients physically in the facility before beginning administration. Vaccinations were commenced on the arrival of all personnel per vial, to avoid wastage in case of failure to attend.
As companies produce more vaccines and the oversight on wasted doses lessens, perhaps the viability of discarding the occasional dose will be available. This problem proved even more relevant when planning for vaccinations at the local potable water factory. It proved nearly impossible to guarantee multiples of 5 or 10 patients. This behoved us to vaccinate as many as possible in one trip due to the numerous other coordination required for movement off-post. Moving forward, we recommend taking plenty of vaccines out to the off-post site and coordinating for ‘filler’ individuals following a return to base. This is a time-consuming process.
The collaboration among different bases was vital as well. While this study excluded vaccine campaigns at outlying facilities, vaccines moved via rotary- wing and fixed-wing assets if frozen. Once thawed, it became limited to ground movements only.
Challenge: Public health campaign
Given the widespread information distribution via social media, delivering a correct and informative message proved challenging at times. Aside from answering questions posed directly to different providers, we developed a few strategies for making ourselves available. The public health campaign focused on information provision, dispelling misinformation and coordination of distribution.
First, we conducted three Town Halls via secure teleconference broadcast throughout the area of operations. Our COVID specialist was dual-trained intensive care and respiratory physician. These sessions delivered a succinct, evidence-based message and opened the broadcast for questions. In addition, we invited a few personnel that had initially been sceptics about the vaccine and had them talk about their experiences and changes in mindset. The use of a stakeholder engagement approach to increase public awareness in vulnerable populations has been described in the US.19,20 Appreciation of disease severity has also been described as a key factor in addressing vaccine hesitancy in a Zambian study.21 Acknowledging that not everyone would be available during the Town Halls, a recording was distributed to further increase messaging.
Second, we addressed the scepticism by meeting personnel in a more suitable environment. The third-country nationals that provide security around the base have limited internet and are subject to poor health information. In conjunction with their leadership, we engaged them during a shift changeover at their place of duty. In this forum, we had direct face-to-face messaging and made ourselves available to correct any misinformation. Misinformation through social media is common and has been noted by skilled nurses and other healthcare workers.22 As with the Town Halls, allowing adequate time for questions was essential. The ability to dispel myths ranging from the risk of developing the disease to the risk of sterility. Promoting accurate information was paramount. The volunteer rate increased from <10% to beyond 90% in this population following 10 such shift changeover meetings.
Third, the Public Affairs Officer created flyers for physical posting and electronic distribution. While the messaging was straightforward, including contact information for further questions directly to physicians proved valuable. Flyers were posted at hand-washing stations in the dining facility, gymnasium and other high-traffic areas.
Finally, the lead operations coordinator is critical in bringing key personnel and information together, and their organisation and central responsibility for managing the list of potentially available vaccine recipients is paramount. Instead of multiple individuals, we recommend a single point of contact to maintain a ‘Master list’, ideally with contact information sent through electronic means. This list was then used to contact individuals and distribute information on vaccine dates and timings, as well as to contact standby personnel to avoid the wastage of doses.
Challenge: Vaccine hesitancy and non- compliance with the vaccination policy
It is important to recognise that vaccination campaigns are unlikely to reach total compliance in any population. The politicisation of the pandemic remained overwhelming, and a minority of individuals refused while it remained a voluntary endeavour. Once mandated by the US Department of Defence (DoD), some US military personnel refused consent, despite the public health campaign mentioned earlier.19,20 The US DoD guidance included advising personnel of their decisions’ potential risks and benefits in a non-judgemental and non- confrontational manner. Though vaccine acceptance is generally higher in military personnel compared to the overall population, the important of clinician- led intervention should not be underestimated.23,24 Vaccination rates in military units can be rapidly improved with effective multidisciplinary vaccination campaigns, as we experienced.25 Though the Israeli Defence Force approach has similarities to ours, their campaign was conducted in established military units in a non-deployed setting. Towards the end of this study period, US DoD mandated vaccination for US military personnel. After due diligence as healthcare providers, any ongoing non-compliance decision was referred to their chain of command per the US DoD guidelines. Vaccinations were offered to coalition partners and local nationals within the catchment area on a purely voluntary basis, following the guidelines of their respective countries.
The strengths of this study are that it details the experience of COVID-19 vaccinations and challenges in a deployed Role 3 facility. There is a paucity of literature on the unique challenges faced in a deployed and austere environment. The military environment produced a captive audience that ensured robust data capture of adverse events. Although in an austere environment, this experience may not fully reflect the additional logistic strain of healthcare facilities further forward, such as smaller Role 2 facilities. A limitation of the study was the availability of medical records within a deployed environment, particularly with coalition partners, contractors and local nationals.
In conclusion, this study highlights the unique challenges of COVID-19 vaccinations in an austere environment. This experience may serve to guide the establishment of future deployed vaccination programs that may be required in future combat deployments and humanitarian emergencies.
Corresponding Author: D.L. Chan, daniel.l.chan@unsw.edu.au
Authors: D.L. Chan1,2,3, D. Fritz 4, M. McMahon5, Peterson6, T. Nessler7
Author Affiliations:
1 Australian Army 2nd Health Battalion – 1/5 Health Support Company
2 Western Sydney University School of Medicine
3 University of New South Wales – Saint George Campus
4 Brooke Army Medical Center – Department of Emergency Medicine
5 Tripler Army Medical Center – Department of Medicinex
6 Brooke Army Medical Center – Department of General Surgery
7 Carl R Darnall Army Medical Center – Department of Emergency Medicine


