Abstract
Haemorrhage control for traumatised soldiers takes place at many levels, from the point of injury through resuscitation and reception into surgical facilities, and postoperatively to intensive care units where normalisation of physiology and ultimate recovery following definitive surgery may be achieved.
Differences in priorities and availability of interventions at each level of care provide unique opportunities for improvement and all contribute towards the ultimate goal of the saving of life with restoration of function and the return of a fit fighting force.
Priorities and challenges at each level are described in this review and are pertinent to the soldier and combat medic on the battlefield, the medical evacuation team providing transport, and the receiving surgical, anaesthetic and intensive care treatment teams stationed at medical facilities in theatres of operation.
Keywords: Haemostasis, trauma
Introduction
Exsanguinating haemorrhage continues to be the leading cause of otherwise potentially survivable acute mortality in current military conflicts1. Two-thirds of lethal haemorrhage is truncal, whereas junctional and peripheral-extremity haemorrhage accounts for roughly 20% and 15% respectively1.
Truncal haemorrhage can only be arrested in adequately equipped medical facilities, but lifesaving techniques can be utilised to control extremity and junctional haemorrhage on the battlefield. Lives can therefore be saved at many points along the continuum of a wounded soldier’s journey from point of injury, through transport to a medical facility and then through the operating theatre and postoperatively to the intensive care unit (ICU) (see
Figure 1).
This review considers haemostasis in a military context from the time of wounding on the battlefield until definitive haemorrhage control and stabilisation in the ICU. It highlights current advances in haemostatic mechanisms available to soldier and combat medic through to surgeon and intensivist in a theatre of operation.
The patient journey from point of wounding and delivery of tactical combat casualty care (TCCC) provided by the individual soldier or combat medic; issues of relevance for transport of bleeding patients; surgical and resuscitative considerations; and, principles of transfusion management and coagulation adjuncts in the ICU will be discussed.
Battlefield haemostasis
Of over 4500 battlefield fatalities prospectively collected by the United States (US) military in the conflicts between 2001 and 2011, the vast majority of deaths occurred on the battlefield. More than one-third of all deaths were categorised as instantaneous (35.2%) and a further 52% occurred within minutes or hours of injury in the pre-medical facility environment. Only 12.5% of deaths occurred once wounded military personnel reached a medical facility1.
Of those deaths occurring prior to arrival at a medical facility, almost 25% were considered potentially survivable; meaning that immediate treatment at the site of wounding is a critical area where effective haemostasis may be lifesaving1. Other studies have shown that up to 20% of haemorrhagic deaths are from compressible bleeding2.
Figure 1: Haemostasis priorities along the continuum of care from point of injury to the intensive care unit
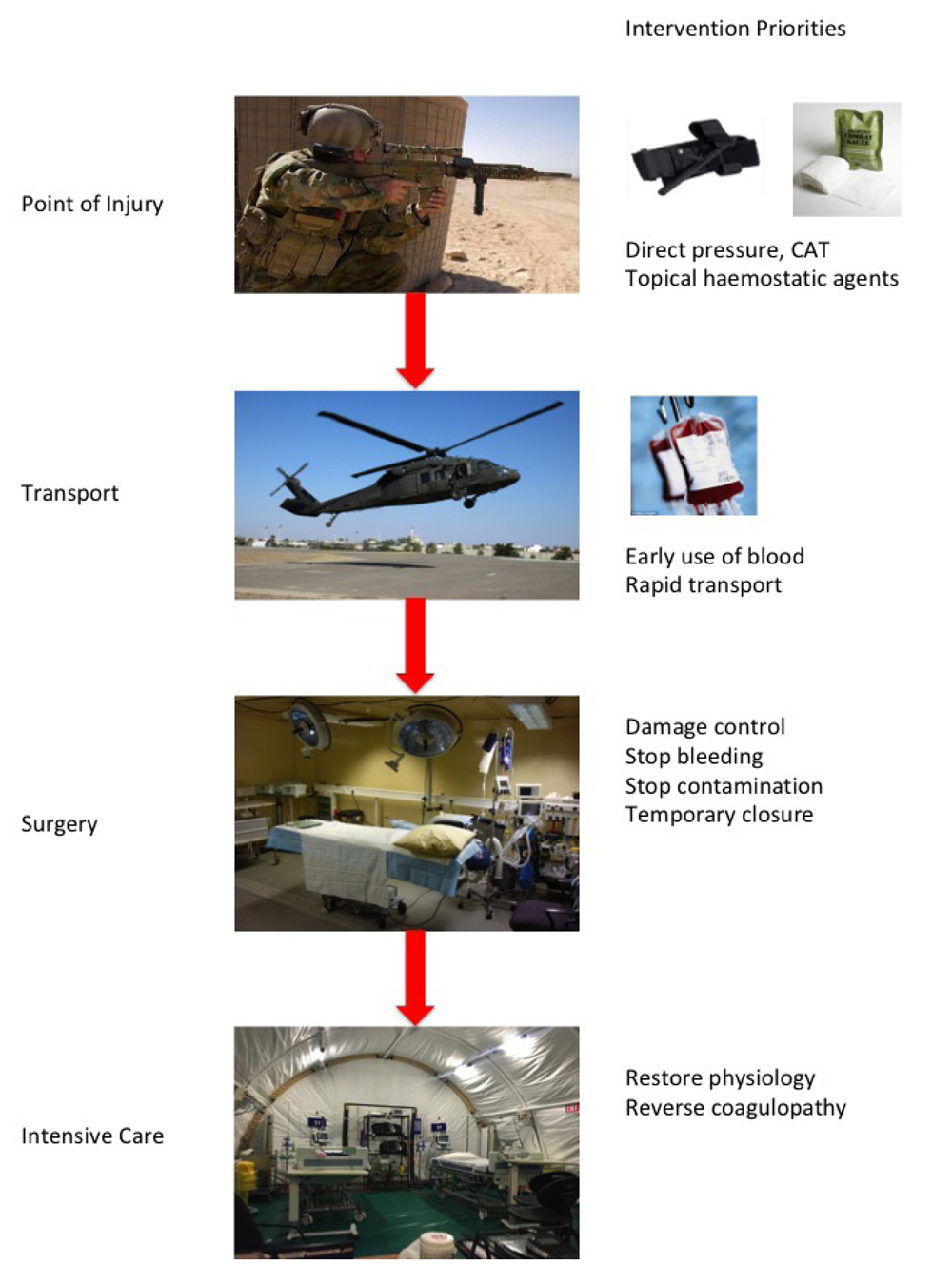
On the battlefield, medical treatment is however, not the only priority. Battlefield casualty care is a combination of good medicine and good small unit tactics. In a broader interpretation of DRABC, danger in the military context often needs to be neutralised by winning the ground battle before efforts at ABC can be employed. Rounds down range often take priority over medical intervention. Whereas in the emergency department, the patient is the mission, on the battlefield, casualty care is only part of the mission. The principles of TCCC have developed and been widely adopted over the last two decades of conflict and direct care at the point of injury on the battlefield3. Simple measures such as direct pressure on arterial bleeding and the early application of the combat application tourniquet (CAT) have the ability to save lives and can be applied by all soldiers in theatre. Advanced wound dressings available to the combat medic also provide additional haemorrhage control at the point of injury. However, extremity haemorrhage still remains the largest preventable cause of death in combat4.
Extremity and junctional haemorrhage
An expert committee formed by the American College of Surgeons Committee on Trauma (ACS-CoT) released guidelines on the prehospital management of severe extremity trauma in 2014 and many of these guidelines have direct military applications or indeed were derived from military experiences5.
Non-truncal haemorrhage can be divided into true extremity haemorrhage and junctional haemorrhage to reflect the different principles of haemorrhage control at these anatomical locations. Junctional haemorrhage was defined as: groin haemorrhage proximal to the inguinal ligament; haemorrhage from buttocks, gluteal and pelvic areas; perineal haemorrhage; axillary and shoulder girdle haemorrhage; and haemorrhage from the base of neck. Extremity haemorrhage reflects bleeding distal to these sites. Major truncal haemorrhage from the chest and abdomen is not covered by these guidelines, the control of which remains in the operating room.
Direct pressure and tourniquets
Direct pressure remains the mainstay of acute extremity or junctional haemorrhage where possible. Tourniquet use is recommended in the prehospital setting where control of significant extremity haemorrhage is ‘ineffective or impractical’ with direct pressure5.
Direct pressure may be impractical under austere conditions with limited resources or multiple casualties. Similarly, in unsecure scenes or where complex extrication or extraction is required, direct pressure may be impractical. In a military context, many of these conditions manifest, and clearly direct pressure under fire fulfils the definition of impracticality4.
Direct pressure can be ineffective in the presence of major (deep) arterial injury, although in wounds associated with significant tissue loss and exposed arterial structures, direct pressure and control remains the gold standard of surgical management. For soldiers and combat medics, a low threshold for activating a CAT has saved lives on the battlefield and the continued use of the CAT is well supported by the available evidence regarding its safety in a military environment.
Before the introduction of tourniquets, the death rate for US forces from peripheral-extremity haemorrhage was 23.3 deaths per year. This was reduced to 3.5 deaths per year after the full implementation of CAT application, representing an 85% decrease in mortality1. Thirty-one per cent of potentially survivable haemorrhagic deaths were from extremity haemorrhage in previous cohorts (reported from previous wars) compared to 13.5% in the current conflicts1 reflecting the impact this simple device has had upon battlefield casualties. Fewer patients are now dying of extremity haemorrhage than seen in previous conflicts and this single advance has revolutionised battlefield haemorrhage control.
Battlefield extrication times have reduced with dedicated airframes for evacuation and in fact, time from wounding to arrival at a level 2 facility can be, in many cases, faster than what can be achieved in a civilian context. As such, concerns about prolonged tourniquet time may be overstated, and the ACS-CoT guidelines recommend not releasing any tourniquet until the patient reaches definitive care5. Exceptions include where transport time is expected to be prolonged, in which case liaison with the appropriate clinical lead is necessary.
Topical haemostatic dressings
Management of junctional haemorrhage on the battlefield is more complex and potentially less effective. The anatomical restrictions of gaining adequate direct pressure are compounded by a similar inability to place a tourniquet proximally.
Topical haemostatic dressings should be used in combination with direct pressure for bleeding from these locations5. The ACS-CoT guidelines recommend using agents delivered in a gauze format that supports wound packing and which can be easily removed surgically (rather than crystalline or powder formulations which are surgically more challenging to disengage). It is important to appreciate that direct pressure remains an essential component of haemostasis when using these products.
The three primary products available for use in a military context are QuikClot® combat gauze, Celox™ gauze and the HemCon™ bandage6. QuikClot was originally produced as a granular form of zeolite powder that absorbed water from the wound, thereby concentrating clotting factors locally. It was best suited for low-pressure bleeding from an unidentifiable source7. In addition to its pure granular form, it came prepared in a gauze-bagged form more suited to removal than the original granules. Initial experience was in the military context, but a report of 46 patients treated in a rural civilian setting with QuikClot also describes effective control of bleeding in 89% of treated patients8. The civilian evidence remains low and of poor quality, and although the results appear promising, an evidence based review from 2013 concluded that further large-scale trials were required9. Additional concerns about the exothermic nature of the original zeolite formulation and secondary thermal burns have led to the current form of QuikClot using kaolin as the active clotting agent impregnated into the gauze dressing rather than zeolite.
HemCon uses chitosan as the active ingredient manufactured from crushed shellfish and promotes haemostasis by the ionic attraction of red blood cells into the dressing causing bonding with the injured tissue surface10. It has been tested in the civilian setting and reported in small series, the largest of which demonstrated it as a safe and effective adjunct in the prehospital treatment of massive external traumatic haemorrhage in 66 patients in the Netherlands11. Complete cessation of haemorrhage was seen in 70% of these patients, with only 6% of cases where correct usage failed to control haemorrhage at all. Celox is similarly a chitosan-impregnated gauze with comparable properties to the HemCon bandage.
All three products are valuable additions to a combat medic’s armamentarium but have training implications for correct usage, as all require proper wound packing and pressure application techniques for optimal effectiveness.
Transport considerations
Following immediate life saving measures to control exsanguinating haemorrhage on the battlefield, the wounded soldier needs to be rapidly and safely transported to higher levels of care where blood replacement and definitive management of bleeding can be instituted. The rotary wing platform provides rapid access and egress forward and is tactical but has some inherent issues of relevance to bleeding cessation, and while en route, the resuscitation efforts must not worsen the patient’s physiology. The acronym GHOSTBaN will be familiar to those trained in rotary wing evacuation and of most relevance to haemostasis are the O, S and T components (Oxygen, the Shakes and Temperature). The fundamental activity of haemoglobin is oxygen carriage and moving to any aeromedical sphere inherently begins to diminish atmospheric oxygen. Similarly with altitude, temperature drops and resuscitative measures are fundamentally undermined by the triad of acidosis, coagulopathy and hypothermia. Keeping bleeding patients warm is a critical element of transport and retrieval that has major flow on effects in terms of resuscitative efforts at higher level care. Finally, the vibrations associated with the rotary wing platform has the potential to cause clot disruption, particularly in the immediate phase post-injury where fibrin cross-linking to stabilise platelet-based clotting has not yet come into full effect.
Damage control resuscitation
Similar concepts of clot preservation underlie the first principle of damage control resuscitation, that is, of permissive hypotension aiming to maintain a systolic blood pressure of 90mmHg but not higher12. This is aimed at preventing renewed bleeding from recently clotted vessels12. The second principle is of volume restoration with what was lost – i.e. a move towards earlier and more aggressive use of fresh frozen plasma (FFP) as the primary resuscitation fluid in addition to packed red blood cell (PRBC) transfusion12. This is manifest in high ratios of plasma and also platelets to PRBC with an aim of 1:1:1 transfusion. Crystalloid is limited to keeping intravenous (IV) lines open between units of blood. The end result is a move away from simply running in huge quantities of crystalloid without thought or endpoint. Rather, it is a considered and measured approach that is much more a balancing act requiring finesse and constant clinical reappraisal to ensure perfusion of vital organs by maintaining sufficient pressure while preserving clot and the body’s own haemostatic mechanisms. The take home message is to minimise crystalloid infusion, accept lower systolic blood pressure and to transport the patient to definitive care for haemorrhage control while minimising periods and extent of hypothermia and without diluting natural coagulation factors.
Surgical techniques
Damage control surgery was first coined in 199313 but the concepts had been published a year earlier14 where 200 patients had been treated over the preceding 7 years with, at the time, ‘unorthodox techniques’ such as the ligation of enteric injuries in 34 patients, retained vascular clamps in 13, temporary intravascular shunts in 4, packing of diffusely bleeding surfaces in 171, and the use of multiple towel clips to close only the skin of the abdominal wall in 178. Since then, the concept has been recognised as the standard of care for the severely injured unstable patient in both civilian and military contexts.
The principles can be summarised as: stop the bleeding; stop the contamination; avoid abdominal compartment syndrome; and prepare for definitive surgery once physiology is restored, (generally somewhere between 48 and 72 hours after initial surgery). There is a shift in emphasis from the operating room to the ICU for stabilisation, with the realisation that prolonged and complex surgery is not safe in patients who are haemodynamically challenged. The minimal activity required to halt further gross anatomical deterioration (i.e. uncontrolled surgical haemorrhage, ongoing enteric soiling from gastrointestinal compromise) is all that is required in theatre, with further attention to restoration of cardiovascular, haematologic, acid–base, thermoregulatory and respiratory parameters taking place in intensive care.
The major threat to life in the immediate instance is uncontrolled haemorrhage. Abdominal bleeding can be categorised as venous, arterial or parenchymal and control of haemorrhage varies slightly in each circumstance. Immediate control of abdominal venous bleeding is most quickly afforded with four quadrant packing, and indeed this may be all that is required in the damage control setting for even substantial venous bleeding. Major venous bleeding from structures such as the vena cava frequently require definitive vascular control much the same as major arterial structures. Smaller arterial structures can generally be ligated, whereas larger structures require repair, bypass or shunt, depending upon the organ supplied. Ultimate proximal vascular control of the abdominal arterial structures can be afforded in desperation via a left lateral thoracotomy and cross-clamping of the descending thoracic aorta, primarily a procedure rarely performed in the emergency department prior to laparotomy. At laparotomy, control of the abdominal aorta in the supracoeliac location as it enters between the diaphragmatic crura has the same effect. In both instances, more localised control at the site of injury must then be attended to in order to minimise secondary injury from occlusion of structures such as the renal arteries and superior mesenteric artery.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has recently been introduced as an emergency department endovascular intervention to control major abdominal or pelvic arterial bleeding in penetrating and blunt trauma15. Balloon inflation achieves the same goal as proximal aortic control during resuscitative thoracotomy with aortic crossclamping without the morbidity and is currently in use in a number of civilian high volume shock trauma centres. Overall, in a study of 285 patients presenting in extremis, almost 10% of patients undergoing REBOA compared with 2.5% of resuscitative thoracotomy patients survived to discharge15. This technique appears to be quite promising and worthy of further study. Improvements in design are now allowing consideration of military field deployability; however, barriers remain in the skillset of front-line providers to achieve arterial access required for REBOA use16.
Management of parenchymal bleeding depends on the organ affected, which may either be resected, repaired or packed as a general principle. For example, although splenic preservation techniques such as buttress suture repair or encasement in vicryl mesh bags went through a phase of popularity at the end of the 1990s and early 2000s, in haemodynamically unstable trauma patients requiring laparotomy and found to have injured spleens, there is now a low threshold for splenectomy. Embolisation in the interventional radiology department for patients with higher grades of splenic injury but who are presently haemodynamically stable is appropriate in many cases not requiring laparotomy and may avoid the need for subsequent surgery.
Parenchymal bleeding from injured livers is mostly effectively managed by packing to approximate anatomical normality and restoration of physiology and coagulation factors in the ICU, but occasionally requires more advanced approaches such as direct suture ligation, or even ligation of major portal inflow structures for control of bleeding. The right or left hepatic artery alone is more likely to require ligation than either the portal vein or the whole portal triad, and this usually means it is not necessary to proceed to emergency hepatectomy at first look laparotomy.
It is likely a combination of packing and selective arterial ligation will be sufficient to achieve adequate haemostasis for transfer of the patient to ICU and upon relook laparotomy and removal of packs, if the liver is sufficiently healthy, nothing further may need to be done (relying solely on portal venous inflow for oxygenation thereafter).
Major bleeding from the limbs is either controlled with simple ligation of smaller vessels or may require arterial repair, shunt or bypass. In severely injured limbs, amputation may be indicated. Damage control orthopaedics follows similar principles to damage control laparotomy in that attention is focused on perfusion and bony stability rather than definitive repair of fractures, and a low threshold for fasciotomy to prevent compartment syndrome and further physiological insult is recommended.
Restoration of physiology and correction of coagulopathy
Following initial surgery for trauma, unstable patients require a period of physiological normalisation in the ICU. Damage control resuscitation principles still apply here and, in particular, high-ratio transfusion of FFP and other products to red cells is appropriate. The use of adjunctive agents has been studied in detail and while some agents are no longer indicated, others appear more promising.
Trauma induced coagulopathy
The traditional view of coagulopathy following trauma has simply viewed consumption of clotting factors and dilution from intravenous fluids and administration of mostly packed red cells as the driving factors, exacerbated by environmental factors, primarily hypothermia and subsequent reduced clotting factor activity.
Our new understanding of trauma induced coagulopathy (TIC) recognises the traumatised patient as being in a hypoperfused state17, with metabolic acidosis, hypocalcaemia and inappropriate breakdown of formed clots by physical manipulation resulting from tissue injury.
Similar to our understanding of the systemic inflammatory response syndrome (SIRS) seen in other critically ill patients, TIC is seen as an overwhelming activation of systems that are usually localised with resultant systemic activation producing whole-body effects, rather than the intended beneficial local actions that usually result from the dual process of clot generation and lysis.
Central to the coagulopathic state is systemic hyperfibrinolysis resulting from massive release of tissue plasminogen activator (tPA) from the endothelium combined with protein C activation and inhibition of the extrinsic pathway of coagulation.
TIC is a highly mortal condition. Of the 135 patients with TIC (defined by INR > 1.2, APTT > 50 sec.) in a cohort of 435 patients undergoing emergency surgery post-injury in whom a massive transfusion was required (>10 units of FFP or PBRC), 53 died (mortality of those with TIC 39.5%)17. Transfusion intervention with a higher ratio of FFP to PRBC was seen to improve mortality in this cohort whereby those patients receiving 1:1 ratio of FFP:PRBC vs those with 1:4 ratio of FFP:PRBC had improved mortality of 28% vs 51% (p<0.03)17.
TIC is a common condition. In another cohort of 106 patients, overall 43% of patients were coagulopathic, with the highest rate in those transfused the most (68%)18.
Appropriate activation of a massive transfusion protocol in these patients is recommended, but there is insufficient evidence to support or refute the use of specific ratios of PRBC to other blood components19.
Adjuncts to coagulation
Despite their use being widespread, the evidence on the use of various blood components in traumatised patients has been poorly studied. When considered individually, there is limited quality evidence on the use of FFP, no studies on the use of platelets or prothrombin complex concentrate or cryoprecipitate. Fibrinogen concentrate has been studied in more detail, primarily in the elective setting, which does not reflect the physiological state of the traumatised emergency patient classically presenting with hypothermia, hypotension and acidosis19.
On the other hand, low fibrinogen has been definitively associated with higher mortality in trauma patients. Despite this, there are no randomised controlled trials on supplementation, type of product (cryoprecipitate, fibrinogen concentrate) or optimal dose19.
There are several products that have been investigated as adjuncts to restoration of normal coagulation in trauma patients.
Prothrombin complex concentrate (PCC) has been used extensively in the reversal of warfarin in the elective surgery setting where its use is established and indicated. Its use in trauma is more controversial and it is not currently indicated as first-line therapy in trauma. It may have a role in refractory bleeding but it is likely inferior or at least not superior to FFP20.
PCC does not contain fibrinogen and although it has the theoretical benefit of not requiring thawing (as FFP does). This can be overcome by having pre-thawed FFP available at trauma centres.
Recombinant Factor VIIa (rFVIIa) initially showed promise as an agent to promote coagulation in extremis and was originally included in part of damage control resuscitation10. However, the available evidence now confirms that the off-label use of rFVIIa in critical bleeding or trauma confers no benefit to mortality outcomes19. In fact, there is a trend towards an increased risk of thromboembolic events and a significantly increased risk of arterial thromboembolic events associated with the use of rFVIIa.
rFVIIa relies on endogenous factor X, II and platelets for efficacy, whereas PCC contains these factors (except platelets) and outperforms rFVIIa in studies that have compared them (which were however, mostly animal studies)20.
It is interesting to note that we appear to have come full circle in our transfusion practices. The first transfusions replaced whole blood, but with further work we came to understand the various components of blood, and separated and transfused these products individually. We now appreciate that traumatic bleeding is not only injurious by lack of the oxygen carrying capacity of red cells, but that coagulopathy associated with the loss of plasma products leads to further ongoing blood loss and other threats to life. Ironically, trauma patients are also at higher risk of thromboembolus post-injury due to loss of antithrombin (and other factors) reflecting the complex interplay of all components of blood. Administration of higher ratios of FFP and platelets to PRBC reflects our appreciation that all elements of blood are critical to normal human life and, in fact, the plasma products may be more lifesaving than simply replacing the red cells that are lost.
The most promising adjunct of recent years is the reintroduction of an old and cheap agent, tranexamic acid (TxA). The important CRASH-2 trial studied 20 211 adult trauma patients with significant bleeding in 274 hospitals across 40 countries21. Patients were randomly assigned (<8 hr post-injury) to either TxA or placebo. All-cause mortality was significantly reduced from 16.0% to 14.5% (1463 deaths in 10 096 administered TxA vs 1613 deaths in 10 115 placebo arm)21. Similarly, the risk of death due to bleeding was also significantly reduced from 5.7% to 4·9%21.
Although this benefit may seem marginal, considering the worldwide impact of trauma, it has been estimated that administering TxA within 3 hours of injury could save over 100 000 lives yearly22.
Highlighting yet again the complexity of trauma coagulopathy and the dynamic physiological changes occurring, TxA administered beyond 3 hours was actually associated with an increased risk of death due to bleeding (4.4% vs 3.1%)23. Nevertheless, this agent and its application in the military setting where first responders are able to reach and treat traumatised soldiers well within the 3 hour timeframe holds promise for the future of battlefield care.
Conclusion
Haemostasis in military trauma should be considered at all points during the continuum of treatment of the traumatised soldier.
On the battlefield, direct pressure, the use of CAT tourniquets for extremity injury and advances in dressings for truncal and cavitating wounds has saved lives. The administration of TxA holds future promise.
During transportation, key factors such as rapid evacuation to higher levels of care, active attention to keeping the patient warm while maintaining an appropriate blood pressure (not simply the highest blood pressure possible) and the early administration of blood, with limitation of clear fluid volume replacement, remain priorities.
In surgery, damage control principles focusing on stopping the bleeding and contamination, while avoiding extended resections and reconstruction are critical.
Postoperatively in the ICU, physiology should be restored and normalised by replacing what is lost prior to definitive return to theatre for completion of surgery intervention.
By optimising each step in the continuum of care, we can expect to reduce soldier mortality from exsanguinating haemorrhage, which remains the leading cause of survivable battlefield mortality.
Conflict of interest
The author states that he has no conflicts of interest.
Corresponding author: Charles Pilgrim, charlespilgrim@hotmail.com
Authors: C Pilgrim1,2,3,4,5
Author Affiliations:
1 The Alfred Hospital
2 Cabrini Hospital
3 Peninsula Health – Frankston Hospital
4 Peninsula Private
5 6HSC, 3HSB






