Abstract
Background: Uncontrolled neuropathic pain can negatively impact quality of life and daily activities. Many treatments have been proven effective, but a significant number of patients still experience uncontrolled pain or intolerable side effects.
Purpose: A small crossover trial has suggested the efficacy of botulinum toxin (BoNT), but the long-term benefits are unknown. This study aims to report the long-term treatment benefits of BoNT in initial responders with refractory neuropathic pain.
Methods: This study identified five male veterans treated with regular intradermal BoNT injection for more than 3 years for painful peripheral neuropathy. The 11-point Likert numeric scale was used to assess neuropathic pain severity before and after the treatments.
Results: One patient had idiopathic neuropathy, while the remainder had diabetic neuropathy. They were initial responders to BoNT and continued injections ranging from 3.5 to 12 years. All reported sustained improvement with an average pain reduction of 7.4 points on Likert scale. No side effects were reported except for mild discomfort associated with the injection.
Conclusion: In this case series, intradermal injection of BoNT every 3 months offers effective, sustained and well-tolerated neuropathic pain reduction in initial responders with either diabetic or idiopathic neuropathy.
Introduction
Diabetes is highly prevalent among adults in the United States and has an even higher rate of occurrence in veterans, with an estimated prevalence of 20.5%.1 Neuropathy is the most common complication of diabetes and affects approximately 50% of diabetic patients. Pain is a frequent complaint and can be debilitating. The exact epidemiology of neuropathic pain is not clearly defined. However, it is estimated that up to 25% of type 2 diabetic patients suffer paraesthesia, described as a prickling and tingling sensation, hyperesthesia or overt pain described as burning, sharp, electric shock-like and lancinating.2 Untreated pain often leads to significant disability, such as reduced physical activity, poor sleep quality, and worsening anxiety and depression.3 These complications can lead to significant financial burdens on patients and the healthcare system.
Neuropathic pain pathophysiology has multiple potential mechanisms that have been investigated but lie with peripheral and central sensitisation.4-5 Peripheral sensitisation is the amplification of peripheral nerve responsiveness. Possible mechanisms include upregulation of nociceptors, altered ion channel function, regeneration of damaged nerves and decreased nerve fibre density. Central sensitisation causes distorted pain perception, possibly through facilitation within the spinal cord’s dorsal horns and the loss of antinociceptive inhibition from the brain.
Sufficient management of neuropathic pain remains a challenge. The American Academy of Neurology (AAN) has a well-published guideline for treatment that includes gabapentinoids, anticonvulsants and antidepressants.6 Despite the availability of multiple pharmacologic therapies, many patients continue to have uncontrolled pain or cannot tolerate side effects. In addition, a 2017 systematic review showed that many medications have highly variable dropout rates.
BoNT has been used successfully in the management of chronic migraine. Two studies have been conducted to evaluate the use of BoNT in treating painful diabetic neuropathy.8-9 Patients in both studies experienced an improvement in their pain; however, neither study examined the long-term responses. This study aims to evaluate the sustained efficacy of BoNT in the responders and explore its potential as a chronic therapeutic option for neuropathic pain.
Methods
The study was exempt by the institutional review board of the Central Virginia VA Health System due to the small number of patients involved and being an observational study. Patients were selected based on a known diagnosis of painful peripheral neuropathy, having failed an adequate trial of standard neuropathic pain medications and initially responding to BoNT.
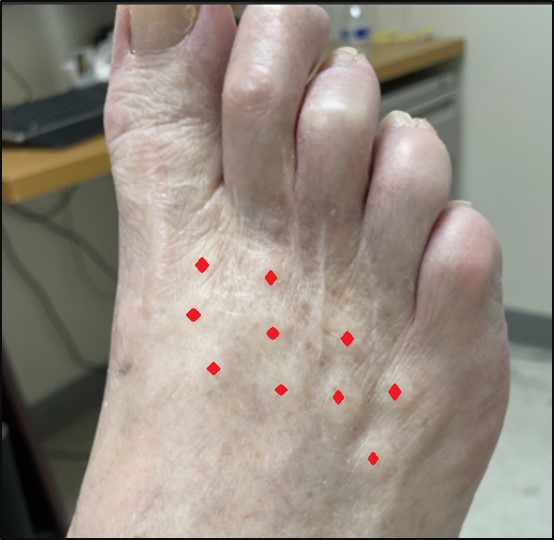
For the injections, we followed a similar protocol as reported by Yuan and colleagues.8 One hundred units of BoNT type A (from Allergan pharmaceuticals) were reconstituted with 2 mL of preservative-free normal saline. The skin of the injection site was cleaned with a 75% alcohol wipe. Fifty units of BoNT were injected intradermally to the distal portion of the dorsum of each foot in approximately 10 sites (Figure 1). The distal portion was chosen due to more severe pain in the toes and better patient tolerance. We monitored pain severity before and after the treatments using the 11-point numeric rating scale along with the presence or absence of night-time waking by neuropathic pain.
Results
Five male veterans were identified for the study. One patient had idiopathic neuropathy, while the remainder had diabetes. Neuropathic pain is the primary complaint in all patients with only minimal large fibre symptoms. The average duration of neuropathic pain was at least 20 years. The pain was described as constant pins-and-needles, a burning sensation with intermittent brief stabbing, electric-shock and shooting pain. The pain predominantly affected the feet, with the most severe pain localised to the toes. The pain severity was rated at 10 by all patients. They stated that it interfered with their mobility and sleep and caused significant distress. All patients had tried and failed five or more conventional agents recommended by the AAN practice guideline. All except one participant took at least one oral medication in addition to BoNT.
Table 1 summarises the key characteristics and responses of all patients. The injections were well tolerated, and all five patients reported notable improvement following the first round of injection, choosing to continue with the treatment due to the significant pain relief. The therapeutic effect was sustained over subsequent treatments ranging from 3 to 12 years. The reduction of pain severity reached more than 60% and seemed to affect the intermittent shooting/stabbing/electric pain followed by the constant burning pain (Figure 2). Most reported a wearing-off period, which often ranged from 10-12 weeks following last injection. At the time of our reporting, all five patients are planning to continue with the treatment.
Interestingly, two of the five patients noted almost immediate ‘relief’ and ‘reduced pressure in the feet’ a few minutes following the injection. The reduction in pain is noted on the same day in three patients, 2–3 days in two patients following the injection.
Neuropathic pain tends to intensify at night. All patients reported reduced waking at night, improved sleep quality and general wellbeing due to better pain control with BoNT injection.
There was no occurrence of skin infection associated with repeated intradermal injection. The injection was safely done even in one patient on oral anticoagulation. The injection was well tolerated, and patients reported no systemic side effects.
Table 1. Clinical characteristics of patients in the study
| Patient | 1 | 2 | 3 | 4 | 5 |
| Age | 90 | 69 | 79 | 71 | 71 |
| Gender | M | M | M | M | M |
| Race | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian |
| Diabetes | + | + | – | + | + |
| Duration of neuropathic pain (years) | 40+ | 20+ | 20+ | 20+ | 20+ |
| Number of medications tried | >5 | >5 | >5 | >5 | >5 |
| Number of current medications | 1 | 2 | 0 | 1 | 1 |
| Pain NRS pre-BoNT | 10 | 10 | 10 | 10 | 10 |
| Pain NRS post-BoNT | 3 | 4 | 2 | 2 | 2 |
| Special note | Most relief of sharp stabbing pain in toes | Stabbing pain is most relieved | Most relief of stabbing pain in feet | Eliminated burning, lightening & stabbing pain | Eliminated stabbing & electric-shock pain & pins-and-needles |
| Night-time waking post-BoNT | infrequent | absent | absent | absent | rare |
| Duration of benefits | 12 weeks | 10 weeks | 10 weeks | 11 weeks | 11 weeks |
| Time BoNT initiated | 2011 | 2009 | 2019 | 2012 | 2018 |
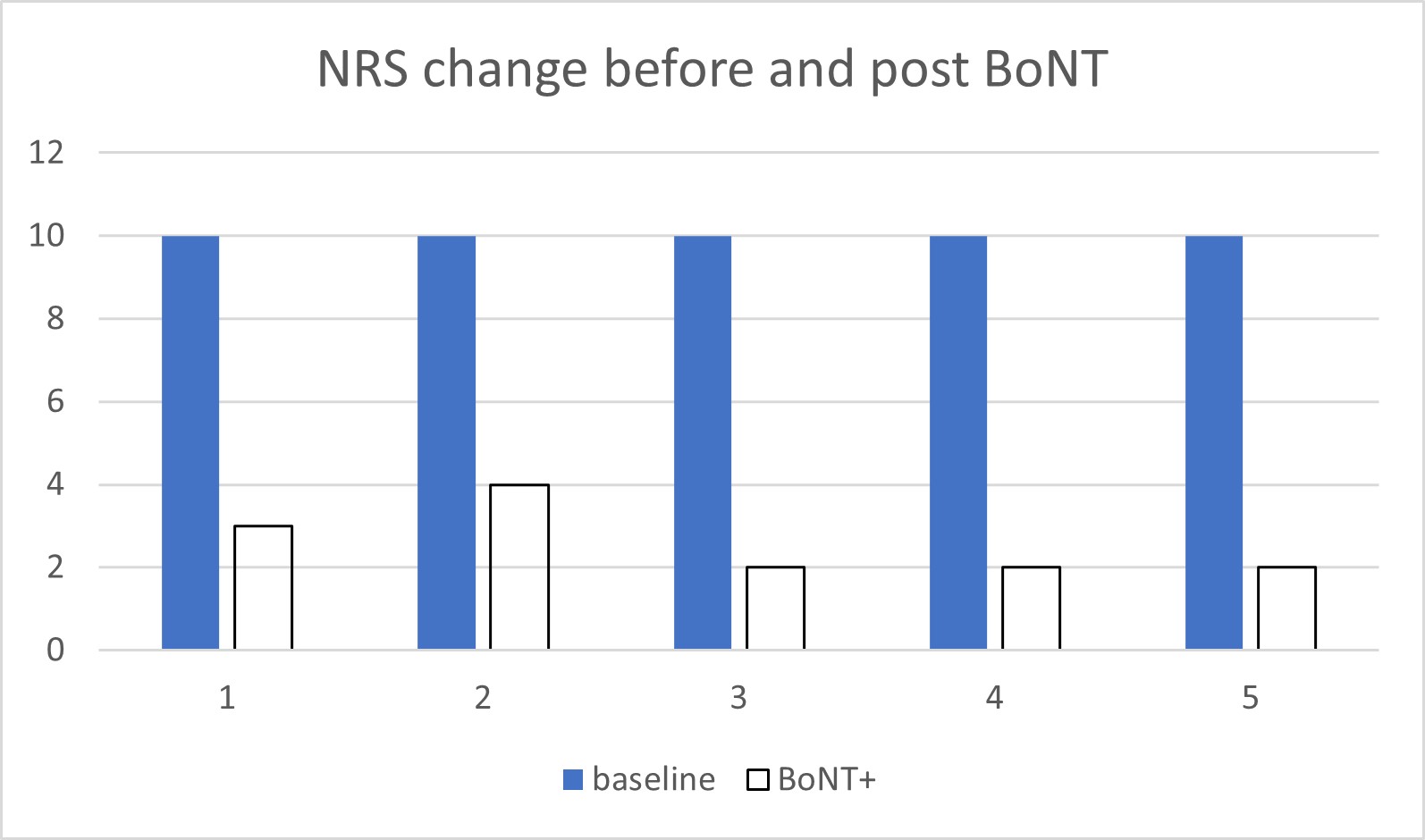
Figure 2
Discussion
This is the first study examining the long-term treatment response of neuropathic pain with BoNT. The study shows sustained benefits of BoNT in relieving neuropathic pain in responders. It supports its use in the clinical management of patients with painful diabetic neuropathy, especially in treatment-refractory patients. The majority of the responders experienced positive responses following the first round of injections and continued to have persistent improvement with subsequent injections, with the longest treatment duration of up to 12 years. The subtypes of neuropathic pain that benefited the most include the sharp stabbing and electric-shock pain, followed by burning and prickling pain. The benefits last from 10 to 12 weeks, with most patients reporting a recurrence of neuropathic pain at a lesser degree by the time of the follow-up injection at a 3-month interval. The benefits do not decrease with repeated injections. The improvement in neuropathic pain positively affected patients’ sleep, quality of life and mood, although not formally scored in the study. No side effects, such as infection, were observed during the treatment, which supports its safety in long-term use. The injection is well tolerated, and the absence of interaction with other medications is a significant advantage.
The results of this study are consistent with the independent trial performed by Yuan et al. in which improvement in pain scale is noted in the treatment group compared to the placebo control following a one-time injection. Furthermore, our study demonstrated sustained improvement in neuropathic pain with repeated injection, opening the door to using BoNT in chronic neuropathic pain management.
Several limitations of our study are recognised. First, this study only included patients whose refractory neuropathic pain improved following an initial trial of BoNT injection. The focus of the study was to examine the sustained benefits, while the exact initial response rate to BoNT was not studied. The small sample size also limits the power of the study due to stringent selection criteria for the initial trial because of the BoNT cost and approval by a local VA pharmacy. However, the positive treatment response is consistently observed with repeat injections (approximately 130). Due to patient selection of only male Caucasian veterans, the extension of the study result to the general population can be limited. Additionally, this single-arm case series study may not provide a confirmative treatment response and eliminate potential placebo effects. However, a unique feature that may help mitigate the potential placebo effects is that the treatment benefits typically wear off 1–2 weeks prior to the next scheduled injection, and the benefits are reinstated following each injection. Another interesting observation is that several veterans had an interruption of BoNT injection during the COVID-19 pandemic. They noted a return of severe neuropathic pain during this equivalent ‘washout’ period.
Although the study showed clinical improvement in the severity of neuropathic pain, the mechanism of BoNT in neuropathic pain remains unclear. The toxin is injected intradermally rather than intramuscularly, so muscle relaxation from inhibited neuromuscular junction is not involved. The relatively rapid onset of action supports peripheral desensitisation through modulating pain mediators or deactivating iron channels at the nerve ending as potential pain relief mechanisms.
Conclusion
The results of this case series demonstrate that regular intradermal BoNT injections provide sustained relief of neuropathic pain in responders. The procedure is well tolerated with no significant side effects. These findings support the long-term use of BoNT in managing refractory neuropathic pain.
Disclosures
We have no financial interests to report for this study.
Corresponding Author: X Du, xinli.du@va.gov
Authors: X. Du1, D. Patel2
Author Affiliations:
1 Hunter Holmes McGuire VA Medical Center Mental Health Services
2 Virginia Commonwealth University – Neurology



