E Daly, N Evans, S Holmes-Brown
Abstract
The purpose of this pilot study was to analyse the current cold chain storage methods of Class 8 stores, specifically thermolabile medications and temperature-sensitive diagnostics, dressings and fluids, for the Australian Army in a training area within Australia. This research was designed to identify deficiencies in current storage methods, including the inability to maintain recommended temperatures of pharmaceuticals in accordance with the Therapeutic Goods Administration (TGA), as well as foster communication between key stakeholders, such as the Royal Australian Army Medical Corps and the Department of Defence Joint Health Command, to develop a cold-chain protocol specific for the Australian Army.
This pilot study identified the common occurrence of breaches in a specific climate, resulting in thermolabile medications, and temperature-sensitive diagnostics, dressings and fluids being commonly exposed to temperatures outside the range recommended by manufacturers. The study’s findings relate mainly to the current storage equipment for Class 8 stores used by the Australian Army and, as a result, recommends the replacement of this mission essential equipment to ensure that cold chain storage meets the TGA guidelines. The study discusses the need for clearly defined guidelines with accountability on the stakeholders to ensure the provision of health support to all Australian Defence Force (ADF) personnel in the field is in accordance with the standard of care expected at a civilian health facility.
Key terms: cold chain storage, thermolabile medications, temperature sensitive, Class 8 stores, storage temperature
Ethical approval and conflict of interest
To ensure objectivity and transparency in conducting this pilot study, the Defence People Research – Low- Risk Ethics Panel was engaged to enquire if any ethical approval was required to conduct this study within the ADF. No ethical approval was required, as the research did not involve humans or animals, but focused on health materiel logistics. There was no identified conflict of interest for the study, which was undertaken by a RAAMC General Service Officer within work time with nil research grants or personal reimbursement provided.
BACKGROUND
Understanding the cold chain
The cold chain is the temperature-controlled aspect of the supply chain and refers to the uninterrupted storage of pharmaceutical products including thermolabile medications, and temperature-sensitive diagnostics, dressings and fluids. The cold chain commences from the time the product is manufactured, it includes transportation to the distribution point, and ends when the product is administered. An unbroken cold chain consists of an uninterrupted series of storage and distribution activities which maintains a given temperature range, based on the manufacturer’s recommended conditions for product stability and integrity stated on the Therapeutic Goods Administration (TGA) approved product packaging.1
National storage and shipping requirements
The cold chain refers to the transportation, storage and distribution of pharmaceutical products within the temperature parameters, which are identified through the product packaging and documentation. The cold chain parameters are outlined in Table 1.
A ‘cold-chain breach’ or ‘adverse storage event’ refers to a situation in which a temperature-sensitive product has been exposed to temperatures outside the specified range for the product – excluding deviations of up to +12°C for less than 15 minutes that may occur when opening the refrigerator door routine use or restocking.3 The implications of a cold-chain breach can have extensive implications on the effectiveness of the product being administered.
When medicines stored at room temperature are exposed to higher temperatures, not only do their physical appearances change4, in some cases, their efficacy and potency can also be reduced.
In accordance with the TGA, medications may be required to remain outside the specific temperature range for a temporary period due to a number of factors, including delivery, processing and refurbishment. Accordingly, Section 8.9 of the TGA states that these temporary breaches of the cold chain must be prevented by all means through forward planning including delivery time, climate and the recommended storage temperature (as per the product label).1 Any new equipment used for the storage of cold chain medicines should be commissioned according to the manufacturer’s written procedure and the storage conditions validated before becoming operational (TGA Sect 8.2).1 There is also the requirement for standard operating procedures (SOPs) to be established to ensure all personnel involved with the cold chain process are aware of what actions to take if a breach occurs, including loss or destruction of the products.
THE AUSTRALIAN DEFENCE FORCE
The Strategic Reform Program
The ADF’s primary focus is to protect and advance Australia’s strategic interests.5 This is achieved through maximising defence capabilities by conducting key training exercises, both domestically and internationally, in order to prepare and deploy personnel to military operations in regions including the Middle East and the South Pacific.
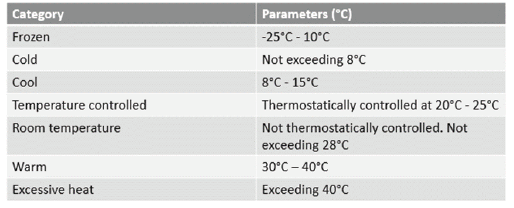
Table 1. Parameters of the cold chain. Reprinted from Reed, C. (2005). COLD CHAINS ARE HOT! Mastering the Challenges of Temperature-Sensitive Distribution in the Supply Chain. Chain Link Research.2
The Strategic Reform Program (2009) was published to strategically plan for and implement changes to maximise the capability of the forces (Army, Navy and Air Force) while creating a more organised and efficient ADF. The overall goal of the Strategic Reform Program is to ‘implement techniques to eliminate duplication and waste in maintaining capabilities, increase their operational availability and reduce the cost of ownership’.6 In order to achieve these goals, three key elements are identified:
- Improved accountability in defence through increasing transparency and accountability of the defence budget;
- Improved defence planning through a strengthened link of planning and military preparedness and refinement of existing governance; and
- Enhanced productivity in defence through improving the cost-effectiveness for military capability and procurement.6
From a logistics perspective, the intent of the reform program is to implement better services and practices to improve the current logistics space. This includes the planning processes, forecasting, waste management and technologies. The end state is that the Department of Defence has improved logistics technologies, increased visibility on the supply chain and generated a return on savings.
Health logistics within the ADF
The ADF currently spends $5.2 billion per year across 23 categories of non-military goods and services from external suppliers.6 Health services are one of these categories and encompass the logistics of pharmaceutical supply and management. The Strategic Reform notes that no less than 60% of any savings on the current budget will be derived from the analysis of any changes to policy, usage and demand.6 As per Figure 1, the costs for health are currently at 100% of the mature yearly savings and need a thorough analysis to consider changes to current practices and processes.
Current state of health logistics in the Australian Army
From the early days of World War I, pharmacists in a military hospital setting were required to work beyond their role with additional responsibilities including the role of Medical Quartermasters. This added duty encompassed stocktaking, auditing, internal checking and writing off damaged or deficient stock.7 Within the Australian Army, logisticians make up approximately 40% of the serving members. Health logisticians are tasked with ensuring the provision of health care is provided to all serving ADF personnel through organic medical assets, which include established medical facilities and field treatment teams and hospitals.
There is a considerable difference in the status quo for logistic management within the Army with the current divide of logistical personnel divided based on their trade or corps and not the ‘level’ of logistics at which one has served in their logistics career.8 This is evident in the current technologies being utilised within the health space, specifically the storage of pharmaceutical products required for the maintenance of the cold chain. The existing cold chain storage methods for close health clinicians, excluding the Haemacol fridges, are not designed specifically for the purpose, but are in accordance with the packing and storage instructions issued by the Land Engineering Agency (LEA) within the ADF and are limited to general-purpose polymer containers.
Role of the Land Engineering Agency
The purpose of the LEA is to ensure that every soldier is delivered equipment that is fit-for-service, safe and environmentally compliant while delivering project outcomes in accordance with the Defence Capability Plan and the Technical Regulatory Framework (TRF).9 The TRF places emphasis on the need for informed (technical) ‘judgements of significance’ to be made by appropriately qualified and competent staff.6 The Packaging, Handling, Storage and Transportation (PHS&T) section of the LEA is responsible for ensuring that materiel system elements within the ADF are preserved, packaged, handled, stored and transported properly until required for use, including ensuring that materiel is protected from climatic, biological and physical hazards and in accordance with the Defence Electronic Supply Chain Manual (ESCM).10 The existing cold chain storage methods highlight the need for significant improvements, required by the LEA and PHS&T, of the fundamental requirements for maintaining the cold chain within the ADF.
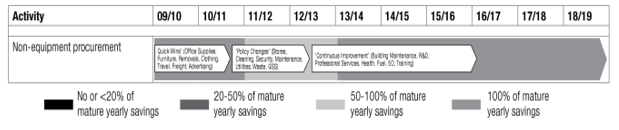
Figure 1. Costs for non-equipment procurement including health services for FY 09/10 to 15/16.
Source: Department of Defence. The Strategic Reform Program. 2009.
Logistics Officers within the Army
Logistics Officers are forced to conduct detailed mission appreciation to ensure that the inflow of Combat Service Support (CSS) sustainment meets planned and unplanned mission demands.11 This planning is within the Army doctrine and while the General Service Officers serving in the Royal Australian Army Medical Corps (RAAMC) are classed as Logistics Officers, the limited training and exposure prior to commencing their roles at the respective health units has not set the corps up for success in regard to the current logistics processes. Furthermore, the lack of process and protocols regarding health logistics, specifically the cold chain management for close health clinicians, do not meet the ‘train the way we fight’ philosophy of the Army.6 Rather, the existing cold chain processes are not enforced in regards to the requirement to replenish and replace any drugs that exceed their recommended storage temperature, as the main effort for the ADF is identified as ensuring all training exercises are executed with limited interruption. Not only does this contravene existing national procedures, but it also increases the risk for all personnel participating in these training exercises.
Maintenance of the cold chain within the Australian Army
This study focused on the current methods employed for cold chain management within the Australian Army, specifically within the 1st Close Health Battalion (1 CHB). The research is being conducted as a result of a previous continuous quality improvement project focused on the storage of all non-refrigerated Class 8 stores during domestic training exercises.
The roles of 1st Close Health Battalion clinicians
1 CHB clinicians are responsible for initial and advanced treatment, including collection from point of injury (POI), resuscitation, stabilisation, and evacuation and emergency diagnostics to land forces as far forward as possible.12 As per Figure 2, this casualty evacuation (CASEVAC) process directs casualties from POI to appropriate health facilities based on required treatment, evacuation platform availability and capacity of the destination medical facility (DMF).
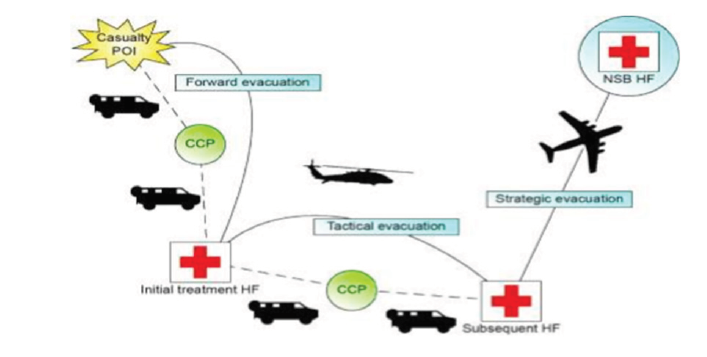
Figure 2. Overview of CASEVAC within the ADF Source: Department of Defence. Defence Health Manual. 2016.
The 1 CHB assets on the ground range from an integral medic at the POI (Figure 3a), an evacuation medic to move the patient (Figure 3b) and a treatment team, comprising of a doctor, nurse and three medics (Figure 3c).13 At all stages of the CASEVAC, the use of thermolabile medications and temperature-sensitive diagnostics, dressings and fluids are required to ensure that health support is effective and adequate.

Figure 3a. 1 CHB Integral Medic responds to a casualty at the POI. Source: Department of Defence. 1st Close Health Battalion. 2018

Figure 3b. 1 CHB Evac Medic loads a casualty in to the Protected Mobility Vehicle (Ambulance variant) for surface evacuation. Source: Department of Defence. 1st Close Health Battalion. 2018

Figure 3c. A 1 CHB Treatment Team stabilises a patient prior to Rotary Wing Aero-Medical evacuation. Source: Department of Defence. 1st Close Health Battalion. 2018
CURRENT 1 CHB STORAGE METHODS FOR THERMOLABILE MEDICATIONS AND TEMPERATURE-SENSITIVE DIAGNOSTICS, DRESSINGS AND FLUIDS
Shipment to the 1 CHB Pharmacy
All pharmaceutical products are shipped from either the Joint Logistics Unit (JLU) in NSW or the prime vendor to the pharmacy in its respective location (Darwin, Townsville, Adelaide or Brisbane).The method of transportation for products requiring refrigeration is a ‘cool cube’; a small expanded polystyrene container designed to maintain interior temperatures of 2°C to 8°C in an ambient temperature range of 5°C to 25°C for up to five days (if the cube remains unopened).The date and time group of when the cool cube was packed is detailed on the exterior of the container with suitable packing material such as foam or cardboard separating the products from the ice packs. A data logger is packed with the pharmaceutical products to continuously monitor the temperature. During transit, the cool cube is only to be removed for aircraft or vehicle stopovers and loading/unloading. It is required to be secured in an air-conditioned building or vehicle if it may be exposed to ambient temperatures outside the 5°C to 25°C range for more than one hour. Once delivered, the pharmacist is responsible for uploading the data logger to ensure that no cold-chain breaches occurred. If the delivery period is not met, or the consignment does not maintain the cold chain, pharmacists are to notify Health Systems Program Office (HLTHSPO) or Joint Logistics Command (JLC), as appropriate, within 24 hours of delivery.(14)
The reporting by the pharmacist includes:
- basic description of the problem including the type of refrigerator or storage method;
- recording the length of time and temperature the stores have been exposed to once internal temperature exceeds 25°C;
- product details and expiry date recorded on the packaging; and
- approximate combined cost of affected stores.
A civilian contractor transports the shipment of thermolabile medications and temperature-sensitive diagnostics, dressings and fluids not requiring refrigeration with no temperature loggers or protocols implemented to report any breaches of storage temperature en route from the prime vendor to the respective 1 CHB pharmacist. The absence of quality control mechanisms is not conducive to the products maintaining their recommended storage temperatures noting the travel times from some vendors to the 1 CHB Pharmacy in Darwin is in excess of 3 days. Furthermore, products requiring shipment from the 1 CHB Pharmacy in Darwin to the 1 CHB Pharmacy in Adelaide, are sent through the Q-Store, which requires an additional 3 days of travel in a storage container without climate control.

Figure 4. Pelican Case used for storage of Schedule 8 drugs by integral medics. Source: Pelican Products, Inc. USA, 2018

Figure 5. Issued trunk for storage of class 8 stores by the Treatment Team. Source: Department of Defence. Land Engineering Agency. 2018.
Field exercises
The clinicians from 1 CHB are required to carry an extensive amount of medical equipment in addition to the basic soldier load list including rifle and ammunition. Dependant on the nature of the training exercise and the unit they are supporting, clinicians will be required to either carry all their equipment on them, have it loaded in an ambulance or be able to self-lift alongside the treatment team with all equipment packed into two vehicles. Specific to the storage of Class 8 supplies, the clinicians store the medications in the following defence issued equipment:
- Integral medic – Integral medics are dismounted and required to carry their Schedule 8 drugs in a Pelican Case (Figure 4). This equipment, measuring 211 mm x 109 mm x 57 mm, is designed for all weather protection and has foam cushioning to protect the medications. It is not able to provide any cooling mechanisms for the medications stored within.
- Evacuation medic – In addition to the Pelican Case, evacuation medics carry a larger volume of medications stored in a standard issue, general-purpose polymer trunk measuring 620 mm x 550 mm x 550 mm. While durable in nature, they are not designed for thermoregulation and are stored in the side of an evacuation vehicle with no air conditioning
- Treatment team – Treatment teams are configured to stock 7 days’ worth of supplies and are required to store these medications in a number of larger general-purpose polymer trunks measuring 1200 mm x 550 mm x 400 mm (Figure 5).They are also equipped with an Engel fridge (Figure 6) in temporary replacement of the Haemacol fridges, which are currently non-serviceable due to ongoing battery issues (which requires action through Chemtronics to JLU).The Engel fridges are not certified to store medicines and are graded for foodstuffs only as they fail to remain between 2ºC to 8ºC without active management, which is not feasible during the road moves from Army bases to training areas (some of which can take four days).Out in the training areas, the access to power is also limited, which affects the ability to provide thermoregulation consistently. The treatment teams are set up in a non-thermoregulated canvas tent, which can experience ambient temperatures of greater than 50ºC when deployed in the hotter months of the year.

Figure 6. Engel fridge carried by the Treatment Team. Source: Engel Australia Pty Ltd. 2016
Medications carried
The medications and diagnostic tests carried by 1 CHB clinicians span a diverse range of required storage conditions and methods of administration. Table 2 provides a number of examples of commonly carried Class 8 stores, which are representative of this diversity and provide the basis for determining conditions that would be considered a breach of recommended storage temperatures in the pilot study.
PRIMARY RESEARCH QUESTION
What are the effects of current cold chain management equipment in controlling the temperature of thermolabile medications and temperature-sensitive diagnostics, dressings and fluids in a routine Australian Army operating/exercise environment?
MATERIALS AND METHODS
Overview
The methodology used in this pilot study was a quantitative statistical analysis through continuous monitoring of ambient temperatures utilising an Environmental Stress Index Monitor (ESIM) and the placement of Tinytags in key storage equipment (Pelican Case, evacuation trunk and treatment team trunk) over a period of nine days within an Australian Army training area. Quantitative research is the preferred approach for testing objective theories by examining the relationship among variables.16 The purpose of this methodology was to identify breaches in the cold chain management through the comparison of real-time data from a variety of different levels of health support and locations within the Townsville Field Training Area (TFTA).
The purpose of this continuous monitoring was to gather definitive data to:
- measure peak temperature;
- calculate the proportion of time medications and equipment spent above the manufacturers recommended storage temperature; and
- record the number of times the temperature exceeded 40°C within the storage containers.

Table 2. Manufacturer recommended storage temperature for scheduled medications and a diagnostic test carried out during EX WARFIGHTER
Sample size
The health support provided during this research was 1 integral medic, 1 evacuation medic and 1 treatment team. This provided an opportunity to measure and compare the temperature of a Pelican Case carried by an integral medic, two smaller trunks carried by an integral medic and evacuation medic and a single large trunk carried by a treatment team. The ambient temperature monitoring by three different personnel (located in different locations within the training area) provided a mean temperature to compare the internal temperatures as well as a contingency if one of the members were to fail to record the temperatures correctly or at all.
Stakeholders
Each stakeholder involved in an analysis is commonly required to have a stake in the phenomenon under investigation.17 It is important to seek the engagement of multiple stakeholders during the planning process to identify and address changes required to improve system effectiveness and efficiency.18 The planning for this research was conducted through the involvement of Army pharmacists, the Battalion Operations Officers, the Senior Nursing Officers and the Senior Medical Officer to devise the terms of reference, key outcomes to analyse and what the proposed end state would be for the pilot study.
Location and time
The data was collected in February and March 2018, at the TFTA located 60km south-west of Townsville and spread out over 200 000 hectares of land. The average temperature during this period is 22–31°C(15) with limited natural cover or shade. This location was utilised due to the timing of a field exercise, EX WARFIGHTER, whereby health support was required for the 800 soldiers deployed to this location for training.
Continuous monitoring systems
The ESIM is a portable heat stress monitoring device which calculates an environmental stress index as an equivalent to a wet bulb globe temperature (WBGT) (Figure 7).19 The waterproof and small design makes it the choice for the Army during training exercises to determine safe work rates. The internal data logging ensures that all data is automatically saved, reducing the requirement for manual recording of temperatures and reducing the risk of the incorrect temperature being manually recorded, or failing to be recorded.

Figure 7. ESIM for recording of ambient temperature Source: Instrulabs Pty. Ltd.

Figure 8. Tinytag logger for recording of storage temperatures. Source: Gemini Data Loggers, UK. 2018
Tinytag loggers are designed for measuring temperature and humidity in a variety of harsh, outdoor and industrial applications (Figure 8).20 Housed in robust, waterproof casings, they are deployed within the ADF for monitoring the storage temperatures of pharmaceutical products, both in barracks and out in the field. The Army pharmacist uploads the data, and graphs and tables portraying temperature changes over a specific period are generated.
The Tinytag was activated and placed in a consignment of refrigerated and non-refrigerated pharmaceuticals for the duration of the field exercise. Ambient temperatures during daylight hours were measured using the ESIM and manually recorded in a spreadsheet. It was also noted if the equipment was in transit and the method of transport. This data was used to compare the internal storage temperatures for the pharmaceutical stores and the impact of the ambient temperature. On return from exercise, the Tinytag data was downloaded and analysed by the pharmacist to determine the continued use or disposal of the refrigerated and non-refrigerated pharmaceuticals.
Continuous monitoring
With these continuous temperature-monitoring results, 1 CHB were able to identify temperature trends through the recorded data of the environments in which the products were stored, while informing on the actual conditions and quality of the current storage methods for the ADF medications. The recording of ambient temperature identified the impact of the training area climate on storage temperatures.
Method for data analysis
Creating ambient temperature profiles was an essential element for this research with the methodical study providing actual data on temperature hazards used to improve storage solutions and advice for the ADF to meet applicable regulatory requirements. The data collected (Appendices A–C) was used to identify the external temperatures experienced throughout the TFTA to provide analysis of the definitive data identified above. This was completed through daily profiling to identify the trends in the ambient temperature and impact on storage methods.
Using Microsoft Excel, each method of temperature collection was given its own recording column. This was used to create the temperature profiles targeting the frequency of occurrences of a particular temperature breach (30°C) and allowed abnormally distributed data to be analysed with a high degree of confidence.
While the data analysis included all hourly temperatures, some temperatures in the observed dataset were deemed not significant in the overall view of the profile and were not considered when creating the climate profiles due to the limited contribution. For example, anything below 25°C and above 41°C was classed as non-significant as these lows/highs contributed to less than 5% of the overall data collected with nil contribution to the profile in a meaningful and representative way. The confidence interval level for this pilot study was 95% as shown in Table 3.
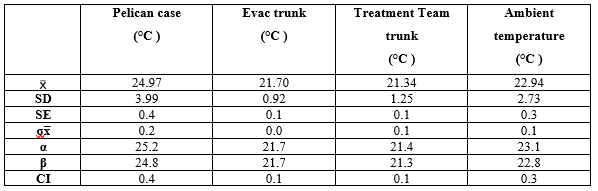
Table 3. Confidence interval levels
With the confidence interval and subsets established, the number of temperature occurrences for the Pelican Case and trunks versus ambient were calculated and a formula of ratios applied. This formula combined the effects of the temperature in the storage containers versus ambient to provide a representative, but usable, set of temperatures. From this, an overall summary was derived and is discussed below.
RESULTS
The analysis was performed with initial profiling created for each day based on the storage method. This was then consolidated to determine the median ambient and internal temperatures for each storage method (Figure 9).The median ambient temperature for this research was 29.4°C with a low of 22°C and a high of 34.9°C.It is noted that at the time of measurement, the region experienced the heaviest rainfall in 12 years,15 which resulted in lower ambient temperatures on some days and increased humidity on others. Overall, the data analysis indicated a correlation between the ambient temperature and the method of storage. The Pelican Case, on average, recorded 3.2°C higher than the ambient temperature and the evacuation trunk and treatment team trunks measured 2.1°C and 1.5°C cooler than the ambient temperatures within their containers. This data was utilised to identify the trend of temperature fluctuations based on the average temperature of the training area in order to identify if the cold chain and supply methods were breached during the training exercise.
Pelican Case
The Pelican Case had a median temperature of 32°C ranging from 24.4°C to 41.8°C. The temperature within the Pelican Case exceeded 30°C on 90 different occasions including exceeding 40°C twice. The percentage of time that the medications were stored beyond the manufacturer’s recommended storage temperatures (25°C–30°C) was 33% for the task duration (Figure 10).
Evacuation and Treatment Team trunks
The data collected for the evacuation and treatment team trunks were similar with a median temperature of 27.6°C (evacuation trunk) and 26.9°C (treatment team trunk).While the temperatures within these trunks did not exceed 30°C, it was consistently over 25°C, which is significant (Figures 11 and 12) given that many of the items stored in these trunks are not recommended to exceed 25°C.

Figure 9. Median temperatures for ambient and storage methods during operation of EX WARFIGHTER
DISCUSSION
Size and dimension implications
While the results showed a number of fluctuations of the ambient temperature, a few key insights and trends were derived from the results including the percentage of time each storage equipment spent within a temperature range and the median heating/ cooling rate of each piece of equipment in response to the ambient temperature.
The first major feature of Figure 9 was the difference between the temperature measured in the Pelican Case versus the evacuation trunk and treatment team trunk. This considerable difference between the three storage methods and recorded temperatures is likely attributed to the size of the container with the Pelican Case, a smaller sized storage method, recording a considerably higher rate of temperatures, consistently exceeding the recommended storage guidelines. This can be linked to a higher heating rate due to the significantly smaller physical dimensions. When considering size as a determinant factor, the evacuation and treatment team trunks recorded almost exactly the same in regards to the management of their internal temperatures and it is assessed that this is due to their similar physical dimensions.
The Pelican Case is also insulated with foam, which may help retain heat in the container. Alternatively, it could be related to where an integral medic stores their Pelican Case versus where the treatment team and evacuation trunks are stored.
Recommendation
For robust statistical analysis to occur, much larger sample sizes deployed in the same training area are required. It is also necessary to assess how each storage method responds when subjected to exactly the same conditions to determine if it is the containers causing the difference or the individual storage conditions.
It is recommended that in future studies a larger number of storage containers are deployed and tracked in the one training area to provide a greater sample size for analysis based on the proportionate time to heat or cool (relative to their size).
Correlation of ambient temperatures
Within the sets of these different geometrics, the derived heating range was used to provide a basic guideline for the impact that the ambient temperature had on the current storage methods. Using Table 4 as a guide, it is hypothesised that current storage methods require review and potential replacement if operating in any environment over 26°C (Pelican Case) or 28°C (evacuation and treatment team trunks) as these temperatures have an impact on the effectiveness of the Class 8 supplies by breaching the recommended storage range (25°C) of most thermolabile medications and temperature-sensitive diagnostics, dressings and fluids carried.
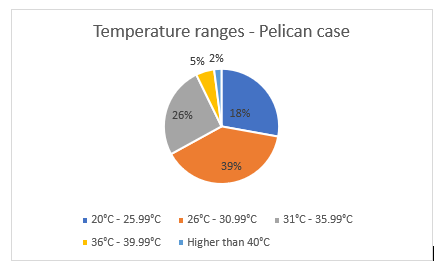
Figure 10. Proportion of time the Pelican Case was measured in each temperature range during operation of EX WARFIGHTER

Figure 11. Proportion of time the evacuation trunk was measured in each temperature range during operation of EX WARFIGHTER
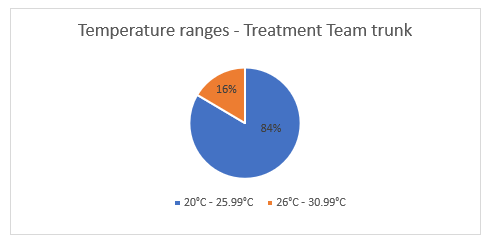
Figure 12. Proportion of time the treatment team trunk was measured in each temperature range during operation of EX WARFIGHTER
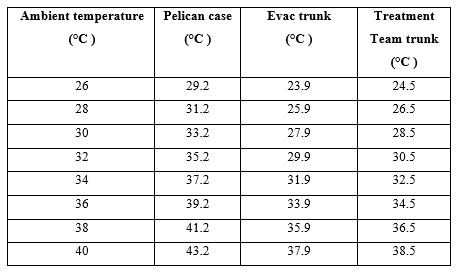
Table 4. Hypothesised correlation of heating / cooling range and ambient temperature
Recommendation
Further studies are required in other ADF domestic training areas including the Northern Territory, NSW and southern Queensland in order to provide a larger sample size for analysis. This data will be imperative to provide a robust discussion on the impact of the provision of health support if this health support is to be deployed to the Middle East Region, which has considerably higher ambient temperatures during summer than Australia, and what the ramifications are if no changes are made to the current storage methods
IMPACT ON THERMOLABILE MEDICATIONS AND TEMPERATURE-SENSITIVE DIAGNOSTICS, DRESSINGS AND FLUIDS
Effect of high-temperature exposure on administration methods
As noted in the results, all thermolabile medications and temperature-sensitive diagnostics, dressings and fluids were exposed to storage temperatures beyond their recognised manufacturer guidelines, which can have adverse impacts on the effectiveness of the medication when administered in a clinical setting. The absence of temperature control can expedite the products to expire in a shorter period of time. (21) The inability for the current cold chain storage methods to maintain this recommended temperature can result in adverse effects when administering health support in remote training areas. Not only can the medications become inactive, but adverse reactions may occur.4
Risk to drug administration
While it is likely that the exposed medications have been compromised in shelf life and efficacy, the true risk of medication degradation and patient safety associated with temperature fluctuations can only be identified and realised with further technical evaluation of these substances and products. The impacts on the medications vary by type, with a number of changes to the potency and effectiveness of administration. Semisolids, including creams like Bactroban, have been shown to exhibit changes in consistency and, as noted by Atia, this can result in a change to how the drug is released.22 Tablets, when exposed to temperatures higher than recommended, can have changes in their appearance due to the disintegration and an increase in density. At low relative humidity, disintegration time is increased, while at high relative humidity disintegration time decreases.23
Recommendation
It is recommended that a separate study be conducted to analyse any changes to the efficacy and potency of the medications that exceed the recommended storage temperature using medications issued to 1 CHB clinicians for the purpose of a task .It is noted that the degradation of biological medicines is not usually amenable to kinetic analysis and extrapolation from accelerated testing(1) which may affect the ability to test certain medications.
Risk to provision of health support
Despite the true risk remaining unidentifiable until further analysis takes place, what is known is that the deployed clinical environment already exposes patients to a higher degree of risk than that within a tertiary health facility. Defence members are already being treated and managed in austere environments with no temperature control and often with junior clinicians who generally have poor access to reliable communications for diagnostic support. These factors, coupled with medications, diagnostic tests and fluids that have potentially degraded due to heat exposure, have the potential to create significant diagnostic confusion and may subsequently result in suboptimal medical management of patients.
As per the Defence Health Manual, any clinical incidents involving ADF members can be reported through a suite of systems to identify the issues with the health care being provided. This includes defined Severity Assessment Codes (SAC) (Table 5) and reporting on clinical incidents and near misses through submissions of an AD441 – Health Incident Report. At all times, clinical incidents that have a work health and safety component which potentially or actually injures a worker or visitor must be reported via Sentinel.12
The regular breach of recommended storage temperatures of thermolabile medications and temperature-sensitive diagnostics, dressings and fluids, and the subsequent impact on the ability to provide effective health care is considered a clinical incident and should therefore, be included as a near miss. When utilising the SAC, the risk to the individual is moderate. However, the likelihood is certain, which demonstrates the requirement for an action plan to be enforced for this issue and tracked by Joint Health Command (JHC) and the ADF Pharmacy and Therapeutics Committee every time a breach of the cold chain occurs.
Recommendation
That an interim solution of cold chain storage methods is employed to assist in the prevention of any future breaches and clinical incidents. Furthermore, it is recommended a review of the JHC SAC is conducted to include cold-chain breaches as a reportable incident and all incidents included for reporting review in all ADF Pharmacy and Therapeutics Committee meetings.
Drug waste costs
In accordance with the TGA (Sect 8.9), if a storage temperature is found to deviate from the manufacturer’s recommended temperature the manufacturer is to be contacted and the suitability of the medicine for use should be determined and the outcome recorded. Furthermore, as per the Defence Health Materiel Manual14 pharmaceuticals must be destroyed and disposed of when storage has affected the integrity of the item. The stock controlling pharmacist is also required to record the destruction of medicines in the pharmacy information system.
If an extreme breach is recorded, the recommended outcome is the destruction of the drug, which will have a financial impact for the ADF. It has been previously demonstrated that inefficiency of drug use and waste production may lead to a distinct economic loss.24 Using the 1 CHB Integral Medic Load List with the HLTHSPO Catalogue, the total cost of replacing all Class 8 stores (excluding consumables such as plasters, bandages and cups) would be $1772.37.These costs include the Pelican Case and the Integral Trunk with the contents of the Pelican Case equalling $280.Noting that the temperature within the Pelican Case exceeded 30°C 44 times over the period of this pilot study, the cost of replacing the contents within the Pelican Case for the duration of the exercise would be $12320 (excluding additional costs of transport and labour to enact these replacements) if the TGA requirements were to be enforced and all medications were deemed unsuitable for use.
As per Item 4 of the ADF Pharmacy and Therapeutics Committee meeting held 13 March 2018, the FY 17/18 Budget Achievement indicates a slight increase over the previous three years of the actual budget and beginning to exceed the forecast budget. Noting that cost analysis above, the budget would continue to be exceeded if the ADF is held accountable by the TGA and these destructions were to occur on a regular basis throughout all ADF training areas.
The SAC includes financial loss as a consideration for assessing the consequences of clinical incidents. The financial loss of less than $100K is considered minor; however, given the frequency of breaches the likelihood is certain.

Table 5. Defence Healthcare Severity Assessment Code (SAC). Retrieved from Defence Health Manual
Recommendation
JHC and the ADF Pharmacy and Therapeutics Committee conduct an analysis into current wastage versus forecasted IAW TGA guidelines of disposal and compare to the costs of purchasing cold chain specific equipment. It is also recommended that the destruction of any medical kits due to a breach of the cold chain be classified as a reportable incident and included for reporting review in all ADF Pharmacy and Therapeutics Committee meetings.
OVERALL RECOMMENDATIONS
While cold chain management can vary greatly in the levels of complexity, there are three principal tenants taken from this pilot study that are simple in nature.
- The current storage methods for thermolabile medications and temperature-sensitive diagnostics, dressings and fluids are not in accordance with the TGA.
- There is a lack of communication, coordination and policy across the ADF Health Logistics space in regards to providing a viable cold chain system that meets national standards.
- There is a lack of monitoring to inform decisions adequately regarding medication disposals.
- Further studies are required in the various training areas to increase data and to provide a more thorough analysis. It is recommended that from these studies, the following objectives be met in conjunction with the Strategic Reform Program and the TGA requirements.
OBJECTIVE 1 – COMMENCE TRIALS OF COLD CHAIN SPECIFIC TECHNOLOGIES OR EQUIPMENT
There are a number of existing specific cold chain technologies and equipment currently employed by coalition military forces and national health organisations that could be trialled to identify a suitable in-service solution; beyond the existing general polymer trunk, for the maintenance of temperature ranges over a prolonged period. This includes both continuous monitoring systems and storage solutions. This trial will require collaboration with the LEA to ensure that the capability is serviceable as per the standards within the Defence ESCM.10
Continuous monitoring – FreshLoc Temperature Monitoring System
Health information systems can significantly bolster cold chain management.25 The FreshLoc Temperature Monitoring System (TMP), used by the US Army, functions by continuously monitoring and recording temperatures of medication storage spaces with the ability to relay the readings wirelessly to the healthcare personnel via text, email or a phone call. This enables temperatures to be tracked continuously and allows healthcare personnel to intervene rapidly to maintain the correct temperature within the storage space. The flexibility and scalability of the system mean that it can monitor any location, from cold storage to incubators, in any size facility26 and could be utilised as a health information system for the ADF and their management of cold chain.
Storage solution – the Cryopak CryoCube
The Cryopak CryoCube, a robust and reusable temperature-controlled product, can sustain a cold chain for prolonged durations ranging from 36 hours to 120 hours (Figure 13).The reusable insulated cube maintains a 2°C–8°C range for 120+ hours. The trunks require insulation panels to be frozen for a minimum 48 hours prior to a task commencing with a variety of sizes available and adequate for the current load configurations of treatment and evacuation teams. The use of this product would reduce the number of cold-chain breaches, specifically during the travel time, and would ensure that the provision of health support is IAW the TGA.
OBJECTIVE 2 – DEVELOP A CLEAR AND CONCISE SET OF SOPS, ENDORSED BY JOINT HEALTH COMMAND AND IN LINE WITH THE TGA, AND IMPLEMENT COLD CHAIN SPECIFIC TRAINING FOR HEALTH LOGISTIC OFFICERS
Delegating responsibility is critical to managing the many moving parts of the cold chain. Army demands from its people, a range of knowledge, a level of competence, and a degree of accountability for actions in the performance of duties, and the involvement of JHC in compiling and endorsing a cold chain directive for all ADF health personnel that will ensure the responsibilities in managing the various complexities of the cold chain are delegated appropriately.
Poor compliance with cold chain assurance is both the responsibility of the individual health care professional and their supporting formation. The creation of a targeted education program for Health Logistic Officers, with a measurable competency for the management of the cold chain, will address the identified issues of health planning, prevention measures for cold-chain breaches, limited understanding of medication management and the processes that must be followed if a cold-chain breach occurs. This will significantly improve the accountability of the Health Logistic Officers in not only maintaining a cold chain in accordance with national guidelines, but through the reduction of risk to the delivery of health care in remote training locations and reducing the cost of waste from the destruction of medications which exceed the manufacturers recommended storage temperatures.
Summary
Meeting these objectives will enable the ADF to qualify and quantify the temperature exposure of the medications and stores and engage with key stakeholders to trial and apply new technologies and processes for the management of the cold chain. This will include working with the TGA to meet the guidelines regarding commissioning of new equipment for the storage of cold chain medicines (Sect 8.2), utilisation of temperature-monitoring equipment that is capable of alerting staff before a temperature range has been compromised (Sect 8.4), and establishment of procedures detailing the actions to be taken in the event of an deviation outside the defined temperature range (Sect 8.9).1

Figure 13. CryoCube Source: Cryopak A TCP Company, 2016
CONCLUSION
The role of the RAAMC is to contribute to the ADF’s operational capability through the conservation of manpower by promoting health and wellbeing, through the prevention of disease and injury, and through the care, treatment and evacuation of sick and wounded.(14) The inability for the current storage methods of thermolabile medications and temperature-sensitive diagnostics, dressings and fluids to maintain the recommended temperature, fails to ensure that the care, treatment and evacuation of ADF personnel in training areas are being achieved. Rather, this initial pilot study has provided evidence that patients are being exposed to higher treatment risks in the field than a civilian health facility. When considering the physical dispersion of Australian Army members within the training area analysed and the proximity of a civilian health care facility being over 45-minute drive away, there is potential for these compromised medications to affect the patient through significant diagnostic confusion. This creates an unacceptable level of risk to patients and commanders and is not in line with the ‘train the way we fight’ theory.
The inability of the Army to meet cold chain requirements for medications relates mainly to a lack of suitable storage equipment. However, poor health planning, complacency and poor understanding of medication management also contribute to the problem. From a risk management perspective, steps need to be taken to address each of these factors. Further research is required in this area to ensure that cold chain management is in line with TGA requirements while meeting the conditions of the Strategic Reform Project.
Corresponding author: Elizabeth Daly, Elizabeth.daly@defence.gov.au Authors: E Daly1, N Evans, S Holmes-Brown Author Affiliations:
1 1st Close Health Battalion
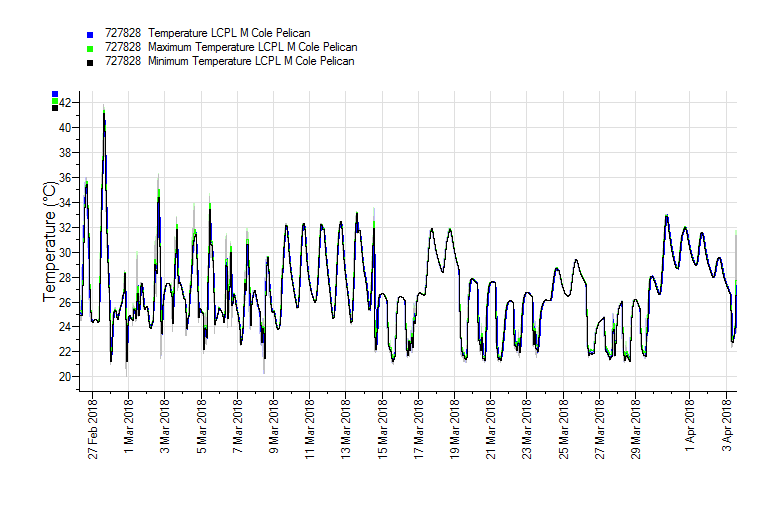
Appendix A – Pelican Case Tiny Tag Results

Appendix B – Evac Trunk Tiny Tag Results

Appendix C – Resupply Trunk Tiny Tag Results






