O Williams, C Lau, V Ross
Abstract
Background: Influenza outbreaks can spread rapidly in confined settings such as military training establishments, impacting operational capability. There are few published examples of influenza outbreak investigations in contemporary Australian military settings.
Methods: An outbreak investigation was conducted in response to an increase in acute respiratory illness (ARI) cases presenting to an Australian military base health centre in June/July 2019. The investigation included a case test-negative analysis and an estimate of the 2019 influenza vaccine effectiveness in the outbreak population.
Results: A total of 66 cases presented during the outbreak; 27 (40.9%) with confirmed influenza cases, 4 (6.1%) with suspected cases of influenza and 35 (53.0%) cases of non-influenza ARI. Those with confirmed influenza infection were significantly more likely to be from the main training unit on base, have a recorded fever over 38oC, and have not received the 2019 influenza vaccine. Cases of confirmed influenza also had significantly more time off work than those with non-influenza ARI. Vaccine effectiveness was estimated at 83% (95% CI = 42% to 95%), with an odds ratio of 0.17 (95% CI = 0.052 to 0.554) of confirmed influenza in those with 2019 influenza vaccination record compared to those without.
Conclusion: This outbreak investigation reinforced the Australian Defence Force’s policy on influenza vaccination. It highlighted the impact that influenza can have on training and work capability, the need for ongoing outbreak surveillance and investigation, and areas for consideration in improving future outbreak control.
Keywords: influenza, outbreak, Australian Defence Force, case test-negative, vaccine effectiveness
Conflict of interest: Dr Olivia Williams plans to include the outbreak investigation in a thesis submission for Masters of Philosophy in Applied Epidemiology (Australian National University). No further conflict of interest declared by any of the listed authors.
Introduction
Influenza is a common viral respiratory illness transmitted from person to person via aerosol, droplet and contact transmission.1, 2 Seasonal epidemics occur most years in Australia—generally over winter, between July and September—but severity, rates and timing vary from year to year, depending on changes in the circulating virus and the population’s susceptibility to it.3
In Australia, influenza outbreaks in closed or confined settings such as aged care facilities,4 prisons,5 schools,6 mass gathering events7 and cruise ships8 have been described in the literature. The impact of the 1918 influenza pandemic on Australian Defence Force (ADF) troops is also well documented,9 and there have been multiple published reports of outbreaks in international military environments.10-17 However, there is limited literature describing influenza outbreaks in a contemporary Australian military setting.
Trends in seasonal influenza illness in the non- deployed military community have been shown to follow those of the surrounding general population,18 although it has been suggested that the confined environment of residential military camps results in earlier epidemic peaks than those seen in the general population.19 When an influenza virus is introduced to the base from an outside source, the close living and working environment, together with training, work or operational conditions, encourage infection transmission.12, 19
Most influenza morbidity and mortality occurs in young children, the elderly or those with comorbidities.1 Published outbreaks in military settings, both in deployed and non-deployed settings, have not reported high rates of complications or hospitalisation.14, 15, 19, 20 However, severe disease and death from influenza can occur in otherwise young healthy adults,21 and even moderate illness can contribute significantly to work disruption and absenteeism.22 In the military setting, this can impact capability and operational readiness, as well as an increased risk of disease spread as the result of a highly mobile workforce.13
Annual vaccination is recommended as the most effective measure for reducing influenza illness and complications at a population level,3 including military populations.11, 23 Influenza vaccination is currently included in the ADF’s routine vaccination schedule and is required for readiness for operations, deployments and major exercises.24 Availability of the 2019 influenza vaccine for ADF members was announced in late April 2019.
The 2019 seasonal influenza epidemic in Australia saw an earlier rise in influenza cases compared to previous years.25 In late June 2019, an Australian Defence health centre reported a considerable increase in possible influenza presentations over the previous 24 hours. An outbreak investigation was initiated with the aim of:
- describing the outbreak epidemiology and determine possible aetiology
- implementing control measures to limit transmission and control the outbreak within the base
- reviewing current Defence outbreak management
This paper describes the investigation of an influenza outbreak at an Australian military base during June/July 2019.
Methods
Study setting and population
The Defence base where the outbreak occurred is located within the metropolitan area of a major Australian city and has a focus on training. There are approximately 1200 personnel working on the base, made up of permanent and reserve Defence members and civilian contractors. During the year, Navy, Army and Air Force trainees pass through the base training establishments. The main education unit on the base (referred to in this paper as the training unit) runs courses ranging from three weeks to 18 months duration.
When attending courses, most of the trainees stay in on-base accommodation in single bedrooms with either a separate or shared bathroom. Common areas include the mess for meals, gym, and education and training areas. Military and civilian staff and contractors generally live off base and commute to work each day.
All Defence members have access to free healthcare, including influenza vaccination. The Defence health centre involved in this outbreak investigation (referred to in this paper as the health centre) is located on base and provides primary healthcare to Defence members who work or study within the base. The health centre, staffed by nursing and medical officers, provides outpatient services Monday to Friday and does not have an inpatient capacity.
During this outbreak, unwell members were clinically managed by health centre staff. Those with possible influenza infection were isolated in their rooms under the care of the training unit staff and medics if living in on-base accommodation, or home if living off base. The isolation period was generally five days from onset of symptoms (in line with public health recommendations2) or until laboratory results returned a non-influenza swab result and the member was well enough to return to duty. General advice regarding respiratory hygiene, seeking care and isolation if symptomatic, and encouraging influenza vaccination was distributed via email to all staff and students on base. The outbreak was followed until 21 July, six days (two usual incubation periods2) following the onset of symptoms of the last confirmed influenza case.
Case definitions
An influenza case was defined as a Defence member who presented to the health centre during the outbreak period with suspected or confirmed influenza, using the Defence policy definitions outlined as follows.
Suspected influenza: A person with onset of respiratory illness from a defined point in time, characterised by fever (temperature >38oC), cough and fatigue.
Confirmed influenza: A person with influenza virus infection confirmed by one or more pathology test.26
Those that presented with respiratory symptoms but did not meet the case definition for suspected or confirmed influenza were classified as having a non- influenza acute respiratory infection (ARI).
Data sources
A line list was commenced by health centre staff following the influx of patients presenting with ARI symptoms on 24 June 2019. Details of presenting cases were added daily while pathology results were pending. Clinical information, including presenting symptoms and date of last influenza vaccination, was collected from the Defence e-Health System (DeHS). Influenza vaccination was considered up-to-date if a 2019 influenza vaccine was received more than
14 days prior to the onset of symptoms, allowing adequate time to develop an immune response. Demographic data about the population who had access to the health centre were sourced from DeHS and the training unit command team provided information about trainees attending on-base courses. Pathology specimens collected by clinicians were analysed at privately contracted laboratories.
The rate of influenza cases was compared with historical rates from the health centre using ADF notifiable diseases surveillance data and with state and national rates over the same period using National Notifiable Diseases Surveillance System (NNDSS) data.
Data analysis
Descriptive analyses were performed on the whole outbreak cohort and each subgroup according to case definitions. Variables included age, sex, student or staff status, unit, living location, date of onset of illness, presentation date to the health centre, symptoms on presentation and influenza vaccination status.
A test-negative design compared confirmed influenza cases with non-influenza ARI controls. Cases that met the definition for suspected influenza were excluded from analysis to reduce misclassification error. Chi-square analysis was used to assess differences between the two groups for categorical variables, and Mann-Whitney U tests were used to compare non-parametric continuous variables.
Crude odds ratios were calculated using binary logistic regression to assess for predictors of confirmed influenza illness. Statistically significant variables were included in multivariate analysis with age and sex to calculate the adjusted odds ratio comparing the odds of 2019 influenza vaccination in confirmed influenza and non-influenza ARI groups. Vaccine effectiveness was calculated using the equation (1 – odds ratio) x 100%.27
Significance was determined at P-value < 0.05. Data analyses were performed using SPSS Statistics 24 (IBM SPSS Statistics for Windows, Version 24.0, NY:IBM Corp).
Ethics
This outbreak investigation was conducted under the authority of Defence Regulation 2016 and Defence Instruction – Administrative Policy and in accordance with the Defence Health Manual.
Results
There were 809 Defence members recorded as attached to the health centre in June 2019, with 43.5% documented as having received a 2019 influenza vaccination. An estimated 400 trainees passed through the base during the outbreak period; however, it is unknown how many of these trainees were vaccinated. Demographics of the population registered with the health centre as of October 2019 (n = 769) are outlined in Table 1 and are assumed to be similar to those of the health centre population at the time of the outbreak.
Descriptive analysis
All ARI cases
The line list consisted of 66 ADF members who presented with ARI during the outbreak period, with a median age of 25 years (range 18 to 52 years) and 81.8% (n = 54) males. Of those included in the line list, 40 (60.6%) were living in on-base accommodation, 42 (63.6%) were trainees, 55 (83.3%) were from the training unit, and 29 (43.9%) had an up-to-date influenza vaccination recorded in DeHS.
The most common symptoms on presentation were nasal congestion (80.3%), cough (75.8%) and sore throat (66.7%). A temperature above 38oC was recorded in 13 cases (19.7%), while 32 (48.5%) members reported symptoms of fever. There were no hospitalisations or severe complications, and none of those who presented with ARI had comorbidities or conditions that increased their risk of complications from influenza. ARIs identified during the outbreak resulted in 213 person-days of sick leave from work with a median of 3 days per person. Only one case was prescribed antivirals based on the clinical decision of the treating medical officer.
Table 1. Demographics and presenting symptoms according to case definition
| Confirmed influenza | Non-influenza ARI | P-value* | Suspected influenza | Health centre population (Oct 2019) | |
|---|---|---|---|---|---|
| Total number n | 27 | 35 | 4 | 769 | |
| Age range (years) n (%) | |||||
| <= 24 | 11 (40.7) | 16 (45.7) | 1 (25.0) | 178 (23.1) | |
| 25–29 | 9 (33.3) | 7 (20.0) | 1 (25.0) | 136 (17.7) | |
| 30–34 | 1 (3.7) | 5 (14.3) | 1 (25.0) | 132 (17.2) | |
| 35–39 | 3 (11.1) | 2 (5.7) | 0 | 101 (13.1) | |
| 40–44 | 2 (7.4) | 4 (11.4) | 1 (25.0) | 84 (10.9) | |
| 45+ | 1 (3.7) | 1 (2.9) | 0 | 138 (17.9) | |
| Age (years) | |||||
| median (range) | 26.0 (19–49) | 25.0 (18–52) | 0.96 | 28.5 (18–43) | |
| Sex n (%) | |||||
| Male | 22 (81.5) | 29 (82.9) | 0.89 | 3 (75.0) | 652 (84.8) |
| Female | 5 (18.5) | 6 (17.1) | 1 (25.0) | 117 (15.2) | |
| Unit n (%) | |||||
| Training unit n | 26 (96.3) | 25 (71.4) | 0.01 | 4 (100) | 380 (49.4) |
| Other units | 1 (3.7) | 10 (28.6) | |||
| Personnel | |||||
| Staff | 6 (22.2) | 3 (8.6) | 0.24 | 2 (50.0) | |
| Trainees | 18 (66.7) | 22 (62.9) | 2 (50.0) | ||
| Unknown | 3 (11.1) | 10 (28.6) | |||
| Accommodation | |||||
| Live on base | 17 (63.0) | 20 (57.1) | 0.64 | 3 (75.0) | |
| Live off base | 10 (37.0) | 15 (42.9) | 1 (25.0) | ||
| 2019 Influenza Vaccination† n (%) | 7 (25.9) | 20 (57.1) | 0.002 | 2 (50.0) | |
| Clinical symptoms reported at presentation n (%) | |||||
| Subjective fever | 15 (55.6) | 17 (48.6) | 0.59 | 0 (0) | |
| Measured fever ‡ | 9 (33.3) | 0 (0) | <0.001 | 4 (100) | |
| Cough | 21 (77.8) | 27 (77.1) | 0.95 | 2 (50.0) | |
| Sore throat | 17 (63.0) | 25 (71.4) | 0.48 | 2 (50.0) | |
| Nasal congestion | 23 (85.2) | 28 (80.0) | 0.60 | 2 (50.0) | |
| Headache | 15 (55.6) | 17 (48.6) | 0.59 | 3 (75.0) | |
| Myalgia/arthralgia | 15 (55.6) | 15 (42.9) | 0.30 | 3 (75.0) | |
| Malaise | 9 (33.3) | 9 (25.7) | 0.51 | 1 (25.0) | |
| Days between onset of symptoms and swab | |||||
| Median (IQR)§ | 2.0 (2.0-3.0) | 2.0 (1.0-3.0) | 0.78 | 0.5 (0.0–1.8) | |
| Days not fit for duty | |||||
| Total person-days | 111 | 87 | 14 | ||
| Median (IQR) | 5.0 (3.0–5.0) | 2.0 (1.0–4.0) | 0.001 | 2.5 (0.8–5.3) | |
* Comparing confirmed influenza and non-influenza ARI
† 2019 Influenza vaccination given 14 days or more prior to onset of symptoms
‡ measured temperature >38oC recorded in clinical consultation notes
IQR = Inter quartile range
Nasopharyngeal swabs were collected from 65 (98.5%) people included on the line list and tested for respiratory viruses by polymerase chain reaction (PCR). The median time between the onset of symptoms and the collection of a respiratory swab for PCR was two days. No other pathology tests for influenza were ordered by clinical staff during this outbreak.
Of those swabbed, 27 (41.5%) were positive for influenza (24 influenza A, three influenza B), 18 (27.7%) were positive for a non-influenza respiratory virus and 20 (30.8%) had negative respiratory PCR swabs. Of those with a negative PCR swab, three met the case definition for suspected influenza infection. One person did not consent to being swabbed but met the case definition for suspected influenza. There were no co-infections detected on respiratory PCR testing and subtype of influenza A infections was not provided. Overall, 27 (40.9%) cases met the case definition for confirmed influenza, 4 (6.1%) met the case definition for suspected influenza, and 35 (53.0%) cases were defined as non-influenza ARI (Figure 1).
The outbreak spanned four weeks, with the onset of ARI symptoms reported from 18 June to 17 July 2019 and presentations to the health centre from 20 June to 18 July 2019. Over half of all ARI cases included in the line list presented to the health centre (54.5%) in the first two weeks of the outbreak (Figure 2).
Confirmed influenza cases
The median age of confirmed influenza cases was 26 years (19–49 years), with 81.5% male, 96.3% from the training unit and 18.5% having received the 2019 influenza vaccination (Table 1). The most common symptoms on presentation in confirmed cases of influenza were measured or subjective fever (88.9%), nasal congestion (85.2%), cough (77.8%) and sore throat (63.0%). Confirmed influenza cases accounted for 111 person-days of sick leave, with a median of 5 days per person. Eighteen (66.7%) confirmed influenza cases presented in the first two weeks of the outbreak (Figure 2).
Confirmation of outbreak
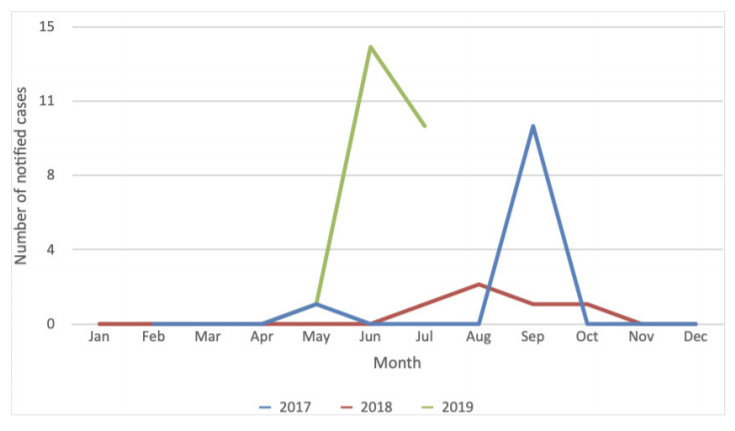
Surveillance data showed an increase in reported cases of confirmed influenza for the health centre compared to the preceding months and the June/ July period in the previous two years (Figure 3). Rates of confirmed influenza from the health centre were calculated using the health centre population in June (n = 809) plus the estimated number of trainees on base during the outbreak period (n = 400). Comparison of confirmed influenza rates with state and national laboratory-confirmed influenza notification rates showed much higher attack rates at the health centre (Figure 4).
Figure 1. Cases presenting with acute respiratory illness (ARI) to the health centre and included in the outbreak investigation
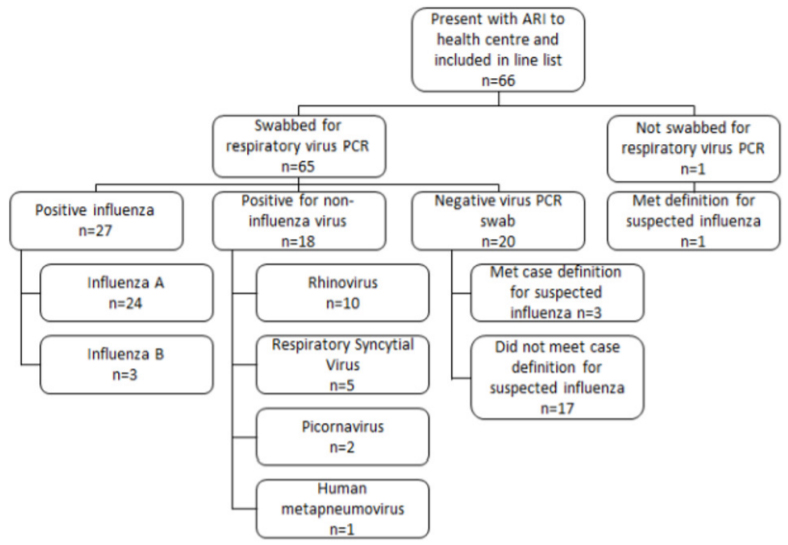
Figure 2. Number of cases according to date of presentation to the health centre and case definition
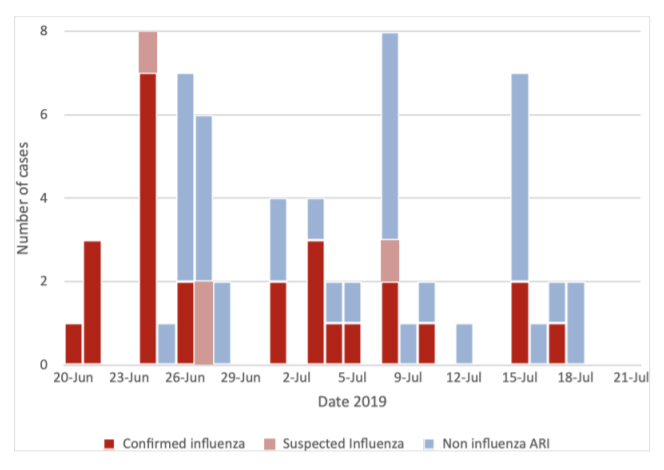
Figure 3. Notified cases of confirmed influenza from the health centre
Comparative analysis
There was no statistically significant difference between influenza and non-influenza ARI groups regarding age, sex, proportion living in on-base accommodation, number of trainees and time between onset of symptoms and respiratory swab collection. Those with confirmed influenza were significantly more likely to be from the training unit (96.3%) and to have a recorded temperature above 38oC (33.3%), compared to those who presented with non-influenza ARI (71.4%, 0% respectively), and less likely to have documentation of having received a 2019 influenza vaccination more than 14 days prior to the onset of symptoms (18.5% vs 57.1%). Those with an influenza diagnosis had significantly more days unfit for work per person (median 5 days) compared to a non-influenza ARI diagnosis (median 2 days).
Figure 4. Reported laboratory-confirmed influenza cases per 100 000 for Australia, state and health centre plus trainee populations
National and State reported laboratory-confirmed cases
Case test-negative analysis
Case test-negative analysis was used to compare the 27 confirmed influenza cases with the 35 influenza test-negative cases. Univariate logistic regression analysis showed that only influenza vaccination status and being from the training unit were significantly associated with confirmed influenza illness. Adjusting for age, sex and unit had little effect on the odds of confirmed influenza in vaccinated compared to unvaccinated members (Table 2). Among ARI cases included in this outbreak investigation, adjusted odds of confirmed influenza in those from the training unit was 11.7 times the odds of influenza in those from other units. Adjusted odds of confirmed influenza in those who had received a 2019 influenza vaccination was 0.17 times the odds of influenza in those who were not vaccinated. Vaccine effectiveness was 83% (95% CI = 42% to 95%) among ARI cases included in this outbreak investigation.
Discussion
This paper documents an investigation into an increase of ARI presentations at a Defence health facility. Pathology testing confirmed an influenza outbreak, primarily affecting young trainees from the main training unit on base. Age and sex distributions of the outbreak cohort are considered to reflect the trainee population on base at the time of the outbreak. Notably, this investigation demonstrated that influenza diagnosis was significantly less likely in those who had a documented 2019 influenza vaccination. It also showed the impact of influenza illness on workforce capability with significantly more days off work due to influenza than non- influenza ARI.
This outbreak investigation demonstrated high vaccine effectiveness when compared with other studies. An Australian general population study calculated an overall 2017 influenza vaccine effectiveness of 39% (95% CI = 24% to 51%) for the 15 to 64-year-old age group.28 Military studies of predominantly young male populations found overall 2009 influenza vaccine effectiveness rates of 46.8% (95% CI = -9.4 to 74.1)16 and 45% (95% CI = 33 to 55%).23 Influenza vaccine effectiveness in the outbreak described in this paper was calculated at 83%; however, the comparatively small study population resulted in low precision with a wide 95% CI (42% to 95%) that overlapped with previous studies’ findings.
Table 2. Odds ratio of confirmed influenza illness
| OR | 95% CI | P-value | aOR | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| 2019 Influenza vaccination | ||||||
| Yes | 0.17 | 0.05–0.55 | 0.003 | 0.17 | 0.05–0.58 | 0.005 |
| No | Ref | Ref | ||||
| From communications training unit | ||||||
| Yes | 10.400 | 1.24–87.31 | 0.03 | 11.669 | 1.23–111.04 | 0.03 |
| No | Ref | Ref | ||||
| Age | 1.001 | 0.94-1.06 | 0.98 | 0.977 | 0.91-1.05 | 0.55 |
| Sex | ||||||
| Male | 0.910 | 0.25–3.37 | 0.89 | 0.78 | 0.17–3.63 | 0.75 |
| Female | Ref | Ref | ||||
OR = Odds ratio
aOR = Adjusted odds ratio
95%CI = 95% confidence interval
Ref = reference group
Vaccine effectiveness from this investigation was based on findings from a single outbreak, constrained in time and location, and without consideration of subtype of influenza A positive specimens. Previous Australian studies have shown that vaccine effectiveness can vary considerably depending on influenza strain and vaccine matching.27, 28 Greater vaccine effectiveness would be expected in outbreaks with influenza virus diversity limited to strains with high vaccine matching. Vaccine effectiveness calculated from this single investigation may not reflect overall vaccine effectiveness to multiple influenza strains and subtypes seen in varied locations and populations over a season.
Strengths of this study include limited confounding variables, presumed high presentation rates to the health centre and high swab rates. Commonly acknowledged confounders in the analysis of vaccine effectiveness include extremes of age, comorbidities and time of year of presentation.27, 28 The young and predominantly healthy population and short study period in this investigation helped minimise these confounders.
Presentation rates of unwell Defence members could be presumed to be high because of easy access to health care, and organisational demands and expectations. However, a perceived culture of shunning weakness, or pressure from training- related timelines that can impact career progression, might also influence health-seeking behaviours in Defence trainees. Other authors have expressed similar concerns about health-seeking behaviours within a deployed military environment.13
High swab rates for investigation of ARI in this outbreak helped reduce misclassification error. While specificity and sensitivity of respiratory PCR are generally high; sensitivity can be affected by poor specimen collection and handling and prolonged time from onset of illness to specimen collection.1 In this investigation, patients tended to present early, with respiratory swabs generally done on initial presentation. There was no significant difference in the number of days from onset of symptoms to respiratory swab between confirmed influenza and non-influenza ARI groups. The high proportion of positive viral PCR tests during this outbreak supports adequate swab quality collected by the health centre.
This study’s weakness was the reliance on clinician discretion for patient investigation and inclusion on the line list rather than according to predefined clinical case definitions. Initially, when clinical staff focused on operational priorities, cases may not have been included in the line list. As the outbreak progressed, the number of presentations and respiratory swabs conducted likely increased as a result of heightened awareness among Defence members and health centre staff. Data on presenting symptoms were collected from clinical notes rather than a standard questionnaire, which may have affected questioning and reporting consistency. Data on vaccination status were obtained from e-health records and, while it eliminates recall bias, relies on good record keeping.
In this outbreak, patients presenting with fever were significantly more likely to be diagnosed with confirmed influenza than a non-influenza ARI. This supports the use of fever in the Defence policy case definition for suspected influenza. Several publications have supported the finding of a temperature above 38oC as a predictor of influenza.1,10 Other studies suggest including reported or subjective fever in the influenza case definition to improve sensitivity;13, 15 however, this is likely to reduce specificity. In this study, there was no significant difference in the proportion reporting subjective fever between those with confirmed influenza and those with non-influenza ARI.
While multiple non-pharmacological public health measures were used to help control the outbreak described in this paper, antiviral prescription was uncommon. Australian public health recommendations focus on antiviral use to treat and prevent transmission of influenza infection in those that are at high risk of complications.2 However, papers have discussed the use of antiviral medication to reduce transmission from infected individuals13 or for post-exposure prophylaxis29 to control influenza outbreaks in confined military settings. Appropriate antiviral prescription can also shorten the infective period, reducing isolation time2 and duration of illness, and may have a role in Defence settings where organisational or operational requirements demand minimal time away from duty.
The primary aim of this investigation was to guide outbreak management. Real-time analysis of findings allowed identification of the cause and the groups most affected and provided the opportunity to influence outbreak control measures, including quarantine of heavily affected training groups, targeted hygiene advice and base-wide promotion of influenza vaccination.
The investigation also provided an opportunity to review the current Defence outbreak management policy. Recommendations resulting from this investigation included ongoing surveillance of influenza cases, outbreaks and vaccination rates to better understand disease burden on Defence; training and support of clinical staff in outbreak management in high-risk Defence settings; encouraging the use the of case definitions to identify cases once an outbreak is established rather than ongoing testing; discussions about the role of antivirals in high-risk Defence settings; and educating commanders on the impact of influenza on, and the role of vaccination in protecting capability. While acknowledging the limitations of the findings from a single outbreak, this investigation supports routine annual influenza vaccination to protect the ADF population from influenza illness, particularly those in confined training and living environments. Ongoing influenza surveillance and outbreak investigation within Defence will enable a complete analysis of influenza infection and vaccination effectiveness across diverse Defence populations in varied locations and over different seasons. Future research should further examine the role of antivirals in helping control influenza outbreaks in these high- risk ADF settings to establish guidelines for antiviral use for transmission reduction and prophylaxis.
Corresponding Author: Olivia Williams olivia.williams@defence.gov.au
Authors: O Williams1, C Lau2, V Ross3
Author Affiliations:
1 Health Policy, Programs & Assurance Banch, Department of Defence
2 Department of Global Health, Research School of Population Health, Australian National University
3 Military Population Health, Health Policy, Programs & Assurance Branch, Department of Defence



