Abstract
Background The purpose of this study was to compare the veteran and non-veteran cohorts of patients diagnosed with bladder cancer in order to determine if veterans have a worse clinical outcome, as has previously been demonstrated in prostate cancer.
Methods Using the Bladder Cancer Outcomes Database at the Repatriation General Hospital, South Australia, all bladder cancer cases between January 1984 and December 2011 were identified. This data was used to identify independent predictors of death in these populations and to contrast their five year bladder cancer- specific and overall survival. A subgroup of muscle-invasive bladder cancer was also analysed. There were a total of 1177 patients studied.
Results Overall, there was no significant difference in bladder cancer specific outcomes for veteran compared to non-veteran subjects. In both groups, the staging of disease at diagnosis was the strongest independent predictor of outcome, followed by the patient’s age at diagnosis. Veterans were generally older at diagnosis than non-veterans, and they did demonstrate worse all cause mortality outcomes.
In the muscle invasive bladder cancer subgroup, outcomes were similar between veterans and non-veterans but veterans were more likely to be treated with radiotherapy.
Conclusion The independent predictors of outcome and bladder cancer specific survival rates in our South Australian cohort were similar to those described in the international literature and do not demonstrate poorer outcomes in our veteran population. All cause mortality was worse in the veteran population, however, which may be related to their older age at diagnosis and different treatments they may be offered as a consequence.
Keywords Bladder cancer, veteran, muscle invasive, survival
Introduction
Bladder cancer is the tenth most common malignancy diagnosed in Australia and has an annual incidence of 2.0% among all newly diagnosed cancers.1 It is the seventh highest cause of cancer related death in the veteran population of the United States.2 It is predominantly diagnosed in older, male patients with almost 80% of patients being over 65 years of age at the time of diagnosis. Since 1984, there has been a decrease observed in the age-standardised incidence of bladder cancer in both male and female Australians, and there has been a corresponding increase in mortality of those who have been diagnosed.3
There is a strong causal link with bladder cancer and tobacco smoking.2-4 A study in 2011 found the risk of bladder cancer to be four times higher in smokers, compared with non-smokers5, and Kuper et al. estimates a decrease of up to 60% in the risk of bladder cancer following cessation of smoking.6 In the United States, it has been shown that the veteran population has a higher prevalence of smoking than the non-veteran population.7 International studies have also demonstrated higher rates of bladder cancer in veterans from the USA and the United Kingdom ; however there are no studies published reporting the incidence of bladder cancer in Australian veterans.2,8
Analyses of the South Australian Prostate Cancer Outcomes Database have demonstrated that veterans have worse clinical disease profiles than non-veterans with prostate cancer, and significantly lower prostate cancer-specific survival rates.9 This study was designed to determine whether a similar relationship exists with Australian veterans and bladder cancer.
A further sub-group of patients with muscle-invasive bladder cancer at diagnosis were identified within the veteran population. Their characteristics, treatment decisions and survival outcomes were compared against a non-veteran population.
Materials and Methods
Ethics approval for this project was obtained from the Southern Adelaide Clinical Human Research Ethics Committee.
The Bladder Cancer Outcomes Database has been maintained at the Repatriation General Hospital in South Australia since 1984, and contains almost 1500 patients. The database records all bladder cancer diagnoses that have been treated at a single public hospital, which caters for both veteran and non-veteran patients within a large catchment area in South Australia. Data is collected prospectively and includes age, gender, disease stage and grade, treatment received, cytotoxic agent use, veteran status and cause of death. The database was originally conceived as a specialist nurse record of patients’ treatment to assist with the administration of intravesical therapy and tracking ongoing surveillance. It has since been approved for research activity.
The Bladder Cancer Outcomes Database was used to identify patients who had been diagnosed between January 1984 and December 2011. Patient data extracted for this study included gender, age at diagnosis, stage and grade at diagnosis, and cause of death. Veterans were defined as patients who held a Department of Veteran’s Affairs Gold Card at the time of diagnosis. Gold Card holders include ex-servicemen and women, and also include war widows and widowers and dependents of servicemen who were killed in the course of duty.10
A subgroup of patient with muscle-invasive bladder cancer were analysed separately using the additional data of American Society of Anaesthesiologists (ASA) score and treatment modality. Comparisons between veteran and non-veteran groups were made. Distribution was compared with Kruskal Wallis tests or a Chi-squared test (Fisher’s exact test where cell counts were low). Means were compared using a t-test. Independent predictors of survival were analysed using a Cox proportional hazards model and five and ten year survival rates were obtained from a Kaplan-Meier curve.
| Descriptors and Outcomes | Veteran status | Totals | Significance p value | ||
| Yes (%) | No (%) | ||||
| Gender | Male | 231 | 671 | 902 | |
| Female | 26 | 248 | 274 | ||
| Mean Age at Diagnosis | 77 | 70 | <0.001 | ||
| Median Follow Up | 61 | 49 | |||
| Five year bladder cancer specific survival (%) | 82 | 85 | |||
| Ten year bladder cancer specific survival (%) | 75 | 72 | |||
| Stage at Diagnosis | Ta | 90 (52) | 390 (56) | 480 |
0.74† |
| T1 | 56 (33) | 197 (28) | 253 | ||
| T2 | 19 (11) | 84 (12) | 103 | ||
| T3 | 5 (3) | 15 (2) | 20 | ||
| T4 | 2 (1) | 12 (2) | 14 | ||
| Totals | 172 (100) | 698 (100) | 870 | ||
| Survival Outcomes | Alive | 79 (31) | 568 (62) | 647 | Reference |
| Death – bladder cancer | 47 (18) | 127 (13) | 174 | < 0.001 | |
| Death – other cause | 127 (50) | 220 (24) | 347 | < 0.001 | |
| Lost to follow up | 0 (0) | 2 (0.2) | 2 | – | |
| Totals | 253 (100) | 917 (100) | 1170 | ||
| Overall Total | 257 | 919 | |||
| Muscle-Invasive Bladder Cancer subgroup | |||||
| Proportion of cohort | 28 | 124 | 152 | ||
| Average Age at Diagnosis | 82 | 74 | <0.001* | ||
| Treatment modality | Surgery | 5 (18) | 52 (43) |
0.002‡ |
|
| Radiation | 17 (61) | 24 (19) | |||
| Palliative | 5 (18) | 35 (28) | |||
| Missing | 1 (3) | 13 (10) | |||
| Totals | 28 (100) | 124 (100) | |||
| ASA status | 1 | 0 | 1 |
0.002† |
|
| 2 | 2 | 33 | |||
| 3 | 18 | 77 | |||
| 4 | 8 | 13 | |||
Table 1: Patient characteristics in overall bladder cancer group and muscle-invasive bladder cancer sub-group
*t-test; † Kruskal-Wallis test; ‡ Chi squared test.
Results
The Bladder Cancer Outcomes Database identified 1466 patients, however only 1176 had veteran status identified and were included in the study. There were 257 veterans and 919 non-veterans. The mean follow up time was 49 months for non-veterans (95% CI 43-54) and 61 months for veterans (95% CI 49-72).
In total, 76.7% of the patient group were male, with a higher proportion of males when comparing the veteran group with non-veterans (90% compared with 73%).
Mean age at diagnosis for veterans was 77 years compared with 70 years for non-veterans (p value <0.001). Almost 80% of veterans were diagnosed between the ages of 70 and 89, whilst the majority of non-veterans were diagnosed from 60 to 79 years of age.
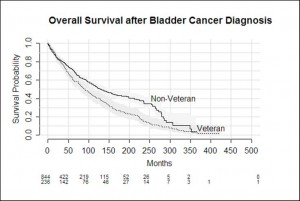
Outcomes recorded in the database for all patients were: Alive, Dead from Bladder Cancer, Dead from Other Cause and Lost to Follow Up. Non-veterans were more likely to be alive. Veterans were more likely to have died from other cause compared with non-veterans (50% compared with 24%, p <0.001). A higher proportion of veterans died from bladder cancer (18% compared with 13%, p <0.001).
Tumour stage at diagnosis was assigned by pathological assessment as Ta, T1, T2, T3 and T4. The tumour stage at diagnosis was similarly distributed between both groups, with no significant differences between the groups. Tumour grade was also evenly spread between the groups, and the p value between the groups was insignificant (p=0.75).
Independent predictors of survival were analysed in a multivariable model and included age at diagnosis, gender, veteran status, stage and grade of disease at diagnosis. Veteran status was not a statistically significant predictor of death from bladder cancer (p
= 0.96). However, age, gender and stage at diagnosis were all significant predictors of death from bladder cancer. Increasing stage at diagnosis was associated with increasingly worse outcomes. The hazard ratio for death from bladder cancer was 27.5 (95% CI 9.4- 80.5) for T4 disease compared to 5.8 (95% CI 2.9- 11.8) for T1 disease.
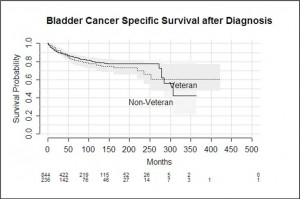
Bladder cancer specific survival was not significantly different between veteran and non-veteran patients (p = 0.58). The longest a veteran lived post diagnosis was 423 months, and the longest a non-veteran lived was for 364 months. Overall survival was significantly worse among veterans compared with non-veterans (p<0.001)
| Variable |
Hazard ratio (95% Confidence Interval) |
P value |
| All Cases – bladder cancer specific survival | ||
| Age at Diagnosis | 1.05 (1.03-1.07) | <0.001 |
| Gender | 1.95 (1.32-2.9) | <0.001 |
| Veteran Status | 1.01 (0.67-1.6) | 0.96 |
| Cancer Stage | ||
| Ta | – | – |
| T1 | 5.8 (2.9-11.8) | <0.001 |
| T2 | 14.8 (6.8-32.1) | <0.001 |
| T3 | 49.5 (20.1-122.1) | <0.001 |
| T4 | 27.5 (9.4-80.5) | <0.001 |
| Cancer Grade | ||
| 1 | – | – |
| 2 | 1.2 (0.4-3.3) | 0.76 |
| 3 | 2.14 (0.7-6.2) | 0.16 |
| Muscle-Invasive Bladder Cancer subgroup – overall survival | ||
| Age | 0.98 (0.97-1.01) | 0.21 |
| Veteran status | 1.36 (0.82-2.26) | 0.23 |
| Gender | 0.61 (0.39-0.93) | 0.02 |
| Treatment Group | ||
| Surgery | – | – |
| Radiation | 3.54 (1.96-6.41) | <0.001 |
| Palliative | 7.01 (3.86-12.76) | <0.001 |
| Missing | 1.33 (0.53-3.33) | 0.53 |
Table 2: Cox proportional hazards model of survival from the time of diagnosis for bladder cancer patients and a subgroup of patients with muscle invasive disease.
Muscle-invasive Bladder Cancer subgroup
A subgroup of patients with muscle-invasive bladder cancer was analysed. One hundred and fifty two patients were identified (28 veterans, 124 non- veterans, p = 0.33), and the average age at diagnosis was 82 years for the veteran group and 74 years for the non-veteran group, which was significantly different (p <0.001). Veterans undergoing surgery had a higher median age than non-veteran patients (78 versus 68.5, p =0.13). There were a higher proportion of male patients in the veteran group.
Veterans were three times more likely to have their muscle-invasive bladder cancer managed with radiotherapy (p <0.001), and were four times less likely to have surgical excision of their bladder compared to the non-veteran group (Table 1). This is most likely reflective of their older age at diagnosis. A similar proportion in each group was managed with palliative intent.
The American Society of Anaesthesiologists (ASA) uses a physical status classification to record patient fitness for an anaesthetic, which ranges from ASA 1, which is assigned to a healthy person, to ASA 4 which is severe systemic disease that is a constant threat to life. The veteran group contained a higher proportion of patients with ASA 3 and 4 (p = 0.02).
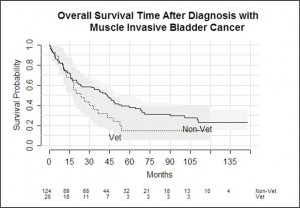
There was no statistically significant difference in survival outcome of muscle-invasive bladder cancer patients between the two patient groups (p = 0.1). Neither age, veteran status nor gender were statistically significant predictors of death in the muscle-invasive subgroup.
Discussion
In this South Australian population, we found that the clinical descriptors of veteran and non-veteran patients with bladder cancer to be similar. The most marked differences occurred in age at diagnosis and gender. The gender difference between the groups was expected, due to the high male proportion within the veteran population.
Smoking history was not reliably recorded in the Bladder Cancer Outcomes Database and was not used for analysis.
Using the DVA Gold Card as the main identifier for Veteran Status does mean that war widows and dependents have been included in our veteran population, however it was not possible to scrutinise their details after de-identification of data. We do acknowledge this as a weakness in our analysis.
Bladder tumour grade was reported by our pathologists according to Mostofi in 1973.11 Modifications to grade occurred in 199812 and 2004,13 and these were also reported to allow for consistent comparison between tumours within the database.
The stage and grade at diagnosis was comparable between the groups. Considering the older age at diagnosis, the equivalent pathological stage suggests that there was no significant delay in diagnosis within the veteran group. The older age at diagnosis may account for the higher proportion of death from other causes in the veteran group. This contrasts with recent studies that demonstrate worse cancer-specific mortality in patients diagnosed at an older age, both because they are diagnosed with higher- risk tumours and are less likely to undergo aggressive treatment.14 We were not able to determine why veterans were diagnosed at an older age than non- veterans.
Female patients in our overall bladder cancer cohort did demonstrate poorer outcomes than male patients. This trend is consistent with international literature.14-15 The reason for poorer outcomes in females requires further investigation, and hypotheses include challenges staging disease within the female pelvis, and delayed diagnosis due to symptoms being attributed to urinary tract infection or overactive bladder. It has also been suggested that women respond less effectively to intravesical treatments, but this is unsubstantiated by evidence.14
Veterans with muscle-invasive bladder cancer were more likely to be treated with radiotherapy than non-veterans, and this is most likely due to older age at diagnosis and poorer anaesthetic fitness. There was no statistical difference between the groups in survival, although the sample size was small.
In conclusion, this South Australian veteran population did not demonstrate a worse clinical disease profile than the non-veteran population. Our cohort was predominantly male and diagnosed at an older age. However there was no statistically significant difference in bladder cancer specific survival, including the muscle-invasive disease
subgroup, compared to the non-veteran group. Veterans did however have worse all cause mortality outcomes. The most significant independent predictor of outcome in all patients is the stage of disease at diagnosis, and this is consistent with the international literature.