Introduction
Post-traumatic stress disorder (PTSD) is a debilitating disorder that affects up to 30% of United States veterans in their lifetime.1-4 Cognitive processing therapy (CPT) is a recommended first-line treatment for veterans with PTSD,5 utilising evidence-based psychotherapy techniques to reduce negative thoughts about oneself and the world, and challenge unhelpful beliefs.6 CPT is widely utilised in the United States Department of Veterans Affairs (VA) for the treatment of PTSD, with cumulative prevalence rates of up to 19.9% recorded between 2001 and 2014.7 The therapeutic process entails a repetitive and detailed exploration of traumatic memories, requiring participants to invest considerable cognitive effort and emotional involvement. Furthermore, the therapy typically extends across multiple sessions, underscoring its comprehensive and time-intensive characteristics, demanding significant resources from both therapists and clients.8
Sleep disturbance, particularly insomnia and recurrent nightmares, is considered one of the most prominent features of PTSD. Additionally, veterans with PTSD face an elevated risk for sleep disorders such as obstructive sleep apnoea (OSA),9 involving repetitive blockage of the airway leading to fragmented sleep and decreased oxygen flow to the brain. Evidence suggests that OSA can exacerbate symptoms of PTSD, and conversely, PTSD may negatively impact symptoms of OSA.10-11 Although the exact reasons for these associations are not entirely clear, the observed higher rates of OSA risk and/or diagnosis in veterans with PTSD may arise from the interconnected effects of chronic stress, combat-related sleep disruptions and the physiological and psychological consequences of PTSD.9 Established OSA risk factors include advanced age, male sex, obesity and high blood pressure,12 but there is increasing evidence challenging the applicability of classic OSA risk factors, especially body mass index (BMI) and age, to young veterans with PTSD. Two recent studies on younger veterans (mean age = 33.4–35.1 years) with lower BMI (BMI = 19.08–28.9) found high rates of OSA risk (63.7–69.2%), suggesting that the relationship between PTSD and OSA may not be fully explained by traditional risk factors within the veteran population.9,13
The significant utilisation of CPT for treating PTSD, coupled with its resource-intensive nature, necessitates an examination of factors influencing its effectiveness. Consequently, it is crucial to pinpoint potential health factors that might affect the efficacy of this treatment approach. Recent evidence suggests OSA is a contributing factor to the reduced effectiveness of CPT for the treatment of PTSD in veterans, as disrupted sleep can adversely impact various aspects of cognitive functioning and learning.14 Continuous positive airway pressure (CPAP) is a primary treatment for OSA that can be effective in increasing restful sleep and oxygenation, as well as reducing daytime sleepiness and nightmares in patients with PTSD. Several studies have reported that CPAP use is associated with improvement in PTSD symptoms.15-19 However, patient adherence to CPAP therapy is often low, particularly among those with comorbid PTSD, which can result in the worsening of both PTSD and OSA symptoms.11,20-21 Thus, improving CPAP adherence becomes a valuable target for enhancing the efficacy of CPT. This retrospective chart review focuses on veterans receiving CPT for PTSD, with the additional consideration of comorbid OSA. This study aimed to investigate whether adherence to CPAP is associated with lower PTSD and depression symptoms in veterans receiving CPT. Results could provide valuable insight into optimising the effectiveness of CPT in individuals with comorbid PTSD and OSA.
Methods
Data source and veteran sample
We identified veterans treated between September 2015 through July 2020. Veterans included were diagnosed with PTSD using the criteria defined within the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), had received at least one session of CPT for PTSD, and were diagnosed with OSA via polysomnography. Further, Veterans were included if they were at least 19 years of age and were issued a CPAP machine for their OSA.
Outcomes
The primary outcome included PTSD symptoms measured via the PTSD Checklist for DSM-5 (PCL-5), with a secondary outcome of depression symptoms measured via the Patient Health Questionnaire-9 (PHQ-9). The PCL-5 is a 20-item self-report measure that assesses the 20 DSM-5 symptoms of PTSD with a total symptom severity score ranging from 0 to 80 obtained by summing the scores for each of the 20 items.22 The PHQ-9 is a 9-item self-report measure based on DSM-5 criteria for major depressive disorder used to assess depression severity with a total score ranging from 0 to 27.23 For both outcomes, higher total scores indicate worse symptom severity. Because this study was primarily focused on response to CPT in veterans with PTSD, the PHQ-9 was not always administered at each CPT session. CPT sessions were ideally delivered weekly for 12 consecutive weeks, but this was unrealistic for many veterans as sessions are time consuming (60–90 minutes) and frequently rescheduled. Additionally, veterans may have forgotten or could not complete the practice assignments requiring sessions to be repeated.
Covariates
Daily CPAP data included mean daily CPAP use, mean residual apnea-hypopnea index (AHI), and mean mask leak. We defined CPAP adherence as ≥4 hours of wear time at night on ≥70% of days between CPT sessions.24-25 We also collected demographic variables that included age, biological sex, race, marital status and BMI.
Statistical analysis
Descriptive statistics are presented as median and interquartile range or count and per cent. Linear mixed-effects models were estimated for PCL-5 and PHQ-9 to account for the repeated outcome measurements from the same veteran. The proportion of outcome variability between veterans was initially quantified via intraclass correlation; we tested heterogeneous veteran-specific residual variances for both outcomes. Time from initiation of CPT to PCL-5 and/or PHQ-9 measurement was quantified in days, with day 0 set at the intake session. For repeatedly measured (e.g., time-varying) covariates of CPAP adherence, daily CPAP wear time, AHI and mask leak, veteran-specific covariate means were included in the model alongside the time-varying covariate to statistically control for veteran-level mean differences across the repeated measurement of the occasion-level covariate (i.e., some veterans averaged higher covariate values across measurements compared to other veterans).26 The functional form for all continuous covariates was estimated using restricted cubic splines with knot points at the 5th, 35th, 65th and 95th percentiles; nonlinear forms were retained as indicated by the likelihood ratio test. Two-way day-by-CPAP covariate interaction effects were estimated to evaluate whether changes in PCL-5 and/or PHQ-9 scores differed by CPAP covariates. All analyses were conducted using SAS vs 9.4 with two-tailed p < 0.05, indicating statistical significance.
Results
Veteran characteristics
We identified 25 veterans during the study period who received CPT for PTSD with access to a CPAP machine for OSA. Veterans had a median of 12 CPT sessions (IQR: 8–12; range: 4–13). Median age was 43 years (IQR: 35–50; range: 26–69), 22 (88%) were male, 24 (96%) were White, the median BMI was 35.6 (IQR: 32.2–41.2; range: 26.7–64.0), and 19 (79%) reported being in a relationship.
PTSD symptoms
During the study period, a total of 261 PCL-5 observations were provided with a median of 11 PCL-5 observations per veteran (IQR: 7–13; range: 2–23) across a median of 125 total days of follow-up (IQR: 83–174; range: 35–549). Approximately 49.7% of the variability in PCL-5 scores was due to veteran-specific differences. Further, differential variability in PCL-5 scores was observed between veterans (–2 log likelihood difference = 2067, df = 24, p < 0.001) that was accounted for by allowing each veteran to have their own estimated residual variance. At the intake session, the average PCL-5 score was 42 (95% CI: 36–49). We observed statistically significant nonlinear change in PCL-5 scores in the days following the intake session (p < 0.001; Figure 1), with improvement in PCL-5 scores beginning around day 125 after intake.
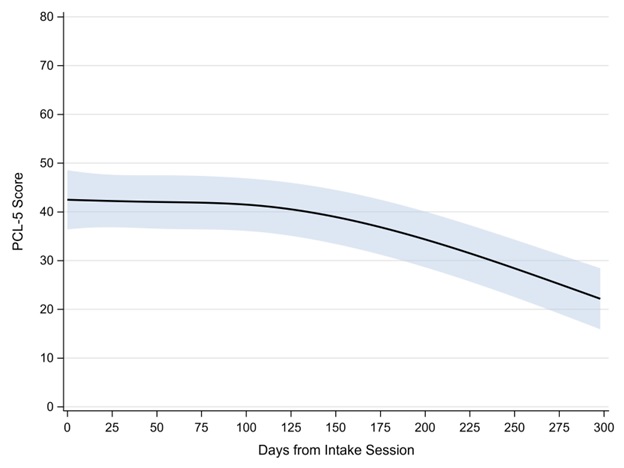
Figure 1. Estimated PCL-5 scores across days since CPT initiation. Shaded area represents the 95% confidence interval.
Concurrent CPAP data were available for 182 (69.7%) of the 261 PCL-5 observations; mask leak data were available for only 127 (48.7%) observations. Table 1 provides descriptive statistics across PCL-5 observations by individual CPAP or OSA covariate. Veterans were adherent to their CPAP use on 48.3% of occasions. After controlling for the proportion of time during which the veteran was adherent with their CPAP, change in PCL-5 scores across the study period did not differ by whether the veteran was adherent with their CPAP between CPT sessions or not (interaction p = 0.668; Figure 2). In addition, statistically similar changes in PCL-5 scores across the study period were observed by CPAP use (interaction p = 0.433), mean residual AHI (interaction p = 0.401), and mean mask leak (interaction p = 0.924).
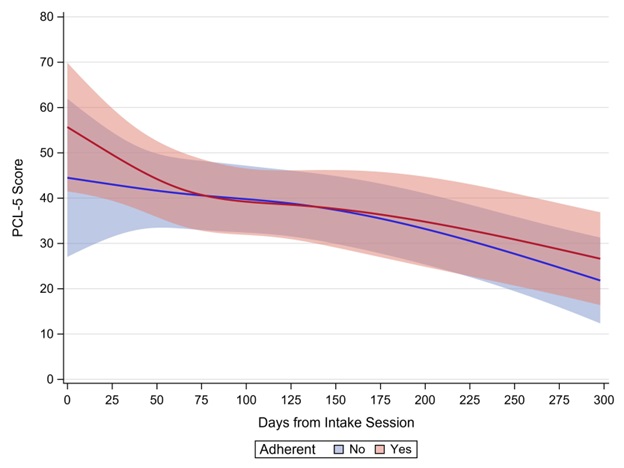
Figure 2. Visual depiction of interaction effect showing estimated PCL-5 score by CPAP adherence. Shaded areas represent 95% confidence intervals. The x-axis was scaled as dictated by having a minimum of 3 veterans providing data.
Table 1. Descriptive statistics for CPAP covariates stratified by outcome
| Covariate | PCL-5 observations (n = 182) |
PHQ-9 observations (n = 130) |
|---|---|---|
| Adherent | ||
| No | 94 (51.7) | 45 (45.9) |
| Yes | 88 (48.3) | 53 (54.1) |
| Overall usage | ||
| No | 55 (30.2) | 26 (26.5) |
| Yes | 127 (69.8) | 72 (73.5) |
| Hours per day | 5.8 [2.4–7.6] | 5.7 [4.0–7.0] |
| AHI | 2.4 [1.5–4.0] | 2.5 [1.9–4.1] |
| Large mask leak | ||
| No | 31 (24.4) | 15 (20.8) |
| Yes | 96 (75.6) | 57 (79.2) |
| Minutes per day | 3.5 [0.9–27.1] | 6.9 [1.0–40.2] |
Note: Descriptive statistics presented as median [IQR] or n (%). ‘Adherent’ defined as CPAP use ≥4 hours per night on 70% of days between CPT sessions.
Depression symptoms
During the study period, a total of 130 PHQ-9 observations were provided with a median of 3 PHQ-9 observations per veteran (IQR: 2–11; range: 1–23) across a median of 107 total days of follow-up (IQR: 80–174; range: 7–549). Approximately 39.3% of the variability in PHQ-9 scores was due to veteran-specific differences; a model allowing differential between-veteran variance across observations would not estimate. At the intake session, the average PHQ-9 score was 14 (95% CI: 11–16). We observed statistically significant nonlinear change in PHQ-9 scores in the days following the intake session (p = 0.021; Figure 3), with improvement in PHQ-9 scores beginning around day 50 after intake.
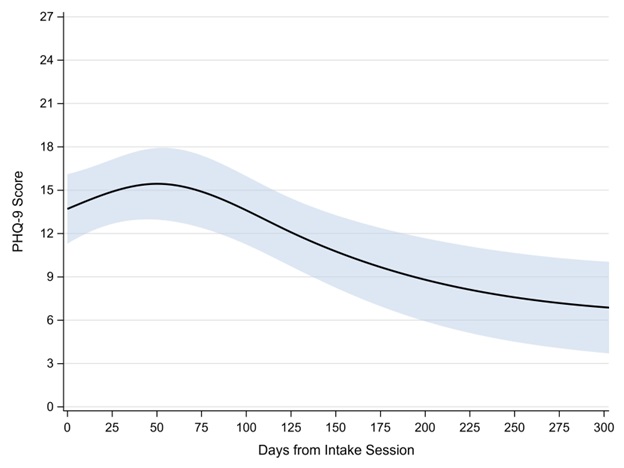
Figure 3. Estimated PHQ-9 score across days since CPT initiation. Shaded area represents the 95% confidence interval.
Concurrent CPAP data were available for 98 (75.4%) of the 130 PHQ-9 observations; mask leak data were available for only 72 (55.4%) observations. Table 1 provides descriptive statistics across PHQ-9 observations by individual CPAP or OSA covariates. Veterans were adherent to their CPAP use on 54.1% of occasions. After controlling for the proportion of time during which the veteran was adherent with their CPAP, change in PHQ-9 scores across the study period did not differ by whether the veteran was adherent with their CPAP between CPT sessions or not (interaction p = 0.124; Figure 4). In addition, statistically similar changes in PHQ-9 scores across the study period were observed by CPAP use (interaction p = 0.447), mean residual AHI (interaction p = 0.768), and mean mask leak (interaction p = 0.881).
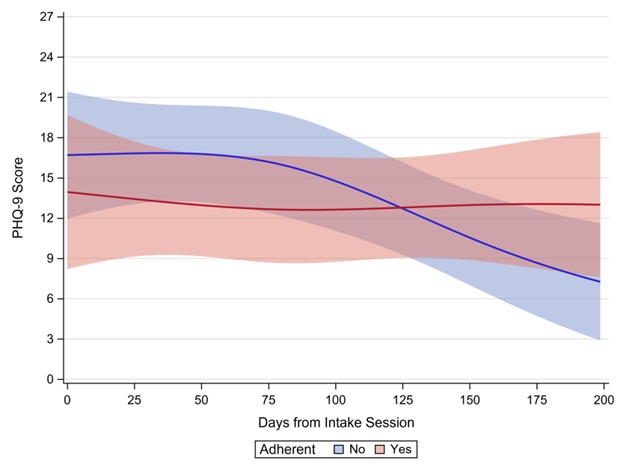
Figure 4. Visual depiction of the interaction effect showing estimated PHQ-9 score by CPAP adherence. Shaded areas represent 95% confidence intervals. The x-axis was scaled as dictated by having a minimum of 3 veterans providing data.
Discussion
We hypothesised that CPAP adherence would be associated with better clinical response to CPT; however, we found that PTSD symptoms improved significantly throughout CPT regardless of CPAP adherence. Similarly, depression symptoms showed significant improvement in both veterans who were CPAP adherent or nonadherent. Further, average hours of CPAP use, residual AHI and mask leak were also not associated with a differential change in PTSD or depression symptoms. Although we did not find statistically significant associations, our study responded to a call to address an important gap in the literature specific to CPT and OSA treatment.14 This is important because, to our knowledge, this is the first study to specifically evaluate CPAP adherence and clinical response to CPT.
There is strong evidence that comorbid OSA increases PTSD symptom severity, as several studies have suggested that CPAP use may play a role in PTSD symptom reduction.11,15,18 Lettieri et al. established that CPAP adherence improved daytime somnolence and quality of life in those with comorbid OSA and PTSD but did not evaluate PTSD symptoms.11 Tamanna et al. found that CPAP adherence reduced nightmares but did not measure overall PTSD symptoms.15 Orr et al. found that the percentage of nights CPAP was used (to any degree) was associated with improvements in PTSD symptoms, but only modestly so.18 None of these investigations evaluated patients undergoing psychotherapies, so our study extends these findings by evaluating the relationship between CPAP adherence and PTSD symptoms throughout psychotherapy.
The efficacy of CPT in PTSD is well established; however, the impact of CPAP adherence on psychotherapy outcomes is relatively unknown.27-28 Mesa et al. found that those with OSA benefited from CPT but to a lesser degree than those without OSA with a secondary finding that those who had ‘presumed access’ to CPAP reported modestly lower PTSD symptoms after CPT than those without CPAP access.14 Inadequate access to CPAP introduces the confounding factor of the ‘healthy user effect’ where individuals with higher utilisation of services (i.e., those that had taken the initiative to seek out and obtain a CPAP device) may be more likely to benefit from non-study interventions such as general health maintenance, pharmacotherapy and social assistance programs. Our study differs in that all veterans had sought out CPAP devices, reducing the ‘healthy user’ effect, and therefore, we were more able to assess the effect of CPAP adherence on symptom modification.
In addition, we found that PHQ-9 scores began to show improvement around day 50 after the initial CPT session. Clinical experience suggests that mild depression symptoms respond faster to psychotherapies than PTSD, and this was the case in our study as PCL scores only started improving at day 125, over 4 months after the intake. This delay in response to CPT might be explained by logistical issues such as scheduling, provider availability, missed appointments and avoidance tendencies that accompany PTSD. Ideally, CPT sessions should be provided at weekly intervals for 12 weeks. A national survey of providers within VA PTSD clinical teams who deliver CPT found significant variability in session frequency with appointments with an average of 3 weeks between sessions.29 Further, it is not uncommon for well-studied CPT protocols used in randomised control trials to fail in translation to clinical practice for various practical reasons such as cancellations of appointments, medical illness, clinic capacity and demand, etc. We did not collect data related to these confounding factors, which could explain the therapeutic lag in PTSD symptoms relative to depression symptoms. Furthermore, the end of our study period overlapped with the COVID-19 pandemic, which likely resulted in significant barriers to CPT session frequency and consistency as clinicians resorted to telehealth.
Our study has limitations inherent to small, retrospective cohort studies, including small sample size, information bias and a limited ability to collect and control confounding factors. We also did not collect data on other sleep and psychiatric conditions, so our results may not represent a population with pure PTSD; it is possible comorbid disorders could have altered CPT response. Further, we could not access polysomnography-derived AHI scores (pre-CPAP use); thus, the AHIs reported in our study are reflective only of residual AHI in those utilising CPAP. As such, the severity of OSA in our sample of veterans could not be determined. This prevented us from evaluating differential CPT benefits by OSA severity. Finally, the veterans in our sample had mild PTSD at baseline and were overwhelmingly male and white. Therefore, our findings could have limited external validity for those with moderate or severe PTSD, female veterans or non-white patient populations. When considered together, our results are best viewed as hypothesis-generating and future research should include a larger sample size with more diverse patients.
Conclusion
Our results suggest that veterans benefit from CPT regardless of CPAP adherence. As such, mental health practitioners should not be discouraged or dissuaded from utilising CPT for veterans who are not adherent to CPAP. However, we recommend that practitioners routinely assess and educate veterans to be compliant with their CPAP, given the adverse health effects of OSA.
Corresponding Author: Sriram Ramaswamy, SriramRamaswamy@creighton.edu
Authors: K Kunes2, C Johnson3, A Kohli1, D Driscoll5,6, R Walters4, S Ramaswamy6
Author Affiliations:
1 Creighton University School of Medicine
2 Oregon Health & Science University
3 Stanford University
4 Creighton University – Department of Clinical
Research and Public Health
5 VA Nebraska-Western Iowa Health Care System
6 Creighton University – Department of Psychiatry


