Methoxyflurane (Penthrox®) was initially introduced as an analgesic into Australian ambulance services in 1974. The last 40 years have seen it become extensively used in all government and nongovernment emergency ambulance services as well as private ambulance providers and patient transport sectors. Its short onset of action and effective analgesic properties makes the drug an important pain relief option in pre-hospital management.
Additionally, methoxyflurane has minimal side effects, including negligible effects on the cardiovascular system and respiratory system, and does not interfere significantly with the examination of the patient on arrival at hospital.1
Methoxyflurane for analgesic use is manufactured and supplied globally from Australia as Penthrox®.
The Australian prescriber information statesthat methoxyflurane is to be administered only to cardiovascularly stable patients, and to those without respiratory depression. This seems to imply that methoxyflurane, even when given in the recommended analgesic doses via the Penthrox® dose limiting inhaler, may decrease blood pressure (BP), pulse rate, or respiratory rate; however this is not reported in clinical practice.
Although there is a large body of published literature supporting the efficacy and safety of methoxyflurane
at analgesic concentrations,2 there is a need for current day supporting studies confirming that this medication does indeed meet modern and regulatory standards, and is safe and effective at analgesic doses.
This is a retrospective, observational study of case records of 590 de-identified ambulance patients. They were investigated in a real life setting (prehospital ambulance transport) to ascertain whether methoxyflurane could be shown to have any impact on cardiovascular and respiratory functions. The study objectives were to ascertain whether any deleterious effects occurred on:
1. Cardiovascular function (systolic blood pressure,
and pulse rate)
2. Respiratory function (respiratory rate)
Methods
St John Ambulance in Western Australia operates the state ambulance service, over an ambulance authority area covering a million square miles – 2.5
million km2 (more than a third of the land area of the continent of Australia). A data collection system using an iPad® has allowed the electronic collation of numerical data. For the purposes of this study, it has provided an accessible mode to retrospectively analyse data on the physiological effects when administering methoxyflurane to ambulance patients in pain.
The Penthrox® inhaler is a hand-held inhalation device (Medical Developments International Limited, Scoresby, Victoria, Australia). The concentration inhaled is variable, depending on individual tidal volume, breathing patterns and airway geometry. However, simulation of in vitro inhalation and exhalation through the inhaler shows that the inhaled concentration of methoxyflurane using the Penthrox inhaler is between 0.1% and 0.7% depending on whether the dilutor hole is open or covered.3 The latter enables an increased concentration of methoxyflurane to be inhaled by patients in more severe pain to provide a faster onset of analgesia. As the methoxyflurane is self- administered, the patient maintains control of their pain management. The maximum quantity available to the subject per dose is 3 mL, i.e. one vial loaded into the inhaler. After an initial loading dose of 6 to 10 breaths through the device (henceforth described as 1 administration), the patient is encouraged to take a few breaths through the device every few minutes thereafter as required. A single vial would only provide analgesia for 20-25 minutes if inhaled continuously, or up to at least 1 hour if used intermittently. A second vial of 3 mL methoxyflurane may be administered when the initial vial has been exhausted.
The data were recorded on the IPad device by the paramedics and volunteer ambulance officers, as part of the patient care process. Staff were aware that the data may be analysed for research purposes. Methoxyflurane was administered under a Clinical Practice Guideline allowing for 3 ml to be given via inhaler, which may be repeated once. It was not practical, or necessary, to determine the exact dose administered during any one episode.
A search of the iPad® data was conducted for all cases that had received methoxyflurane between 15 August 2011 and 4 April 2012, and had at least three sets of observations for systolic BP, pulse rate, and respiratory rate, and where there was no other record of analgesic use. Although this may have biased the selection of the data, three sets were chosen to establish a trend, and to establish whether a single out-of-limits reading was supported by the others, or was just an entry error. Values that were below the normal limits that we set for the 3 parameters were analysed separately:
1. Pulse rates that were lower than 60 beats/min,
or above 100 beats/minute.;
2. Systolic BP readings that were lower than 90
mm Hg, or above 140 mm Hg;
3. Respiratory rates that were lower than 11
breaths/minute, or above 30 breaths/minute.
These limits are similar to those quoted for average values in healthy people, and are widely accepted, with minor variations.4,5
It is recognised that many healthy individuals have resting rates outside these figures.
The recorded brief patient incident history was also reviewed as necessary for confirmatory information. For each patient, mean values for systolic BP, pulse rate and respiratory rate were calculated for 4 time periods: before methoxyflurane administration, 0-10 minutes after administration was initiated, 11- 20 minutes after administration was initiated, and 21-30 minutes after administration was initiated. Henceforth, all references to ‘before’ and ‘after’ methoxyflurane administration refer to before and after the start of methoxyflurane administration.
Results
Patient Disposition
Only patients with 3 or more observations were included in the analysis, which was carried out using SAS version 5.2. Five hundred and ninety patients fulfilled this criterion and were included in the study, with over 2063 valid individual sets of observations recorded for these patients.
Most patients included in this report had 3 sets of observations of their vital signs carried out (396 patients, 67.1%). One hundred and twenty four patients had 4 observation sets, (21.0%), and most of the remainder had 5 or 6 sets, with 7 patients (1.2%) having over 6 sets of observations.
The majority of patients received 1 administration of methoxyflurane (551 patients, 93.4%). Thirty eight patients received 2 administrations of methoxyflurane, representing 6.4% of the total. One patient (0.2%) received 3 administrations of methoxyflurane.
Patient Demographics
The mean age of the patients was 50 years (SD: 24 years), and they ranged from 5 years of age to 99 years of age. The majority of patients were 18 years of age and over (537 patients, 91.1%) with 47.8% (282) aged 50 and above, and 19.8% (117) over the age of 75 years. The ratio of male to female patients transported by ambulance over the last complete year were 47% male: 58% female. The ratio for those who received methoxyflurane was 44% male: 55.9% female. This probably reflects the greater number of surviving females to males in the old, illness and injury- prone age group.
A summary of patient demographics by indication is given in the Table.
INSERT TABLE HERE
Just over half the patients presented with trauma (302 patients, 51.2%). Trauma comprised mainly domestic trauma (injuries received in the home),
but also included sporting injuries, motor vehicle accident trauma, assault- related injuries, industryrelated injuries, and undefined trauma. The next
most common indications were musculoskeletal problems (118 patients, 20.0%) and abdominal problems (86 patients, 14.6%).
Overall Changes in Vital Signs
Systolic BP
Figure 1 shows the difference in systolic BP before and after methoxyflurane administration.
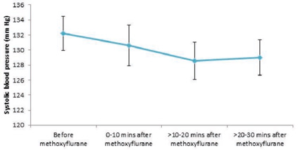
Overall, systolic BP fell slightly after methoxyflurane administration from a mean of 132.2 mm Hg (SD: 23.9) to 130.6 mm Hg (SD: 23.7) 0 to 10 minutes after administration, and then plateaued to around 129 mm Hg between 10 and 30 minutes after administration. All mean values across all patients for each time period were well above 90 mm Hg and within normal limits. (110-140mm Hg).
Nearly all patients (>95%) both before and after methoxyflurane administration had levels of systolic BP within normal limits. The proportion
of patients that had ‘abnormal’ values (both above and below our defined normal levels) decreased after methoxyflurane administration, and there was no evidence that methoxyflurane inhalation increased the probability of exhibiting abnormal systolic BP.
In general, there was no particular pattern to the patients’ systolic BP levels after methoxyflurane administration across age classes, other than an
initial decline, associated with relief of suffering. Additionally, there was little overall pattern in systolic BP levels among patients presenting with different indications after methoxyflurane administration. There was, however, a greater early decrease in systolic BP after methoxyflurane inhalation in cardiac patients and some with unspecified illnesses, before levels settled at their previous levels within 30 minutes of inhalation. However, much of this large initial decrease can be accounted for by individuals with extreme baseline values; for example, one cardiac patient had a systolic BP of 220 mm Hg before methoxyflurane inhalation decreased the elevation associated with the patients initial physiological response to pain.
Pulse rate
Figure 2 shows the difference in pulse rate before and after methoxyflurane administration.
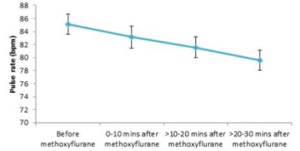
Overall, pulse rate fell continuously after methoxyflurane administration from a mean of 85.1 beats/min (SD: 16.8) to 79.6 beats/min (SD: 13.5)
between 20 and 30 minutes after administration. All mean values across all patients for each time period were well above 60 beats/min and within our range of normal resting limits.
Most patients (>80%) both before and after methoxyflurane administration had pulse rates within normal limits. The proportion of patients that
had abnormal values (both above and below our defined normal levels) decreased after methoxyflurane administration, and the proportion of values within normal limits increased steadily between 0 and 30 minutes after methoxyflurane inhalation. There was no evidence that methoxyflurane inhalation increased the probability of exhibiting abnormal pulse rates.
When considering methoxyflurane administration by age classes, the sharpest decrease was found in children under the age of 12; pulse rates in these
patients fell by approximately 10 beats/minute within 10 minutes of methoxyflurane administration, and pulse rates remained around that level until at least 30 minutes after methoxyflurane administration.
There was little overall pattern in pulse rate among patients presenting with different indications after methoxyflurane administration. There was, however, a relatively sharp and immediate decrease in pulse rate up to 30 minutes after methoxyflurane inhalation in patients who had had environmental related injuries (such as bites or stings). Another unusual observation was that pulse rates increased after methoxyflurane inhalation in geriatric patients or those with debility. However, all changes were small and were within normal limits.
Respiratory rate
Figure 3. shows the difference in respiratory rate before and after methoxyflurane administration.
Figure 3. Mean (95% CI) respiratory rates before and after methoxyflurane administration
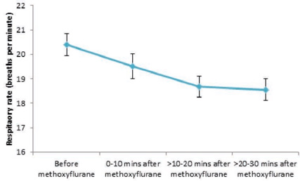
Overall, respiratory rate fell slightly after methoxyflurane administration from a mean of 20.4 breaths/minute (SD: 4.9) to 18.6 breaths/ minute (SD: 3.9) between 20 and 30 minutes after administration. All mean values across all patients for each time period were well above 10 breaths/ minute and within our normal limits (12 – 18 breaths/minute for adults).
No patient had a respiratory rate of less than 10 breaths/minute before or after methoxyflurane inhalation. Most patients (>88%) both before and after methoxyflurane administration had respiratory rates within normal limits. The proportion of patients that had abnormal values above normal levels decreased after methoxyflurane administration, and the proportion of values within normal limits increased steadily between 0 and 30 minutes after
methoxyflurane inhalation. There was no evidence that methoxyflurane inhalation increased the probability of exhibiting abnormal respiratory rates.
In general, respiratory rate decreased slightly across age classes after methoxyflurane administration, except for a rise in adolescents from 19 breaths/minute before methoxyflurane to 21 breaths/minute within 10 minutes of receiving methoxyflurane. However, there was a great deal of variation in these patients as indicated by the large standard deviation, so it is likely that a handful of outliers may have caused this aberrant result. Patients in most age
classes exhibited a stabilising of their respiratory rates after 10 minutes of receiving methoxyflurane, except children under the age of 12 whose respiratory rates continued to decrease 20-30 minutes after methoxyflurane administration.
Consistent with other vital signs measured, there was little overall pattern in respiratory rate after methoxyflurane administration among patients presenting with different indications. An unusual result was the large increase in respiratory rate up to 10 minutes after methoxyflurane inhalation in geriatric patients or those with debility, although there was significant variation around the mean in this indication group. However, all changes were
small and were within normal limits.
Lower than normal Values in Vital Signs A subset of the data was extracted that only included patients with any systolic BP values of less than 90 mm Hg, a pulse rate of less than 60 beats/min, or a respiratory rate of less than 10 breaths/minuteafter methoxyflurane administration. The lower values exhibited after methoxyflurane inhalation
were compared with their values before inhalation, to ascertain whether there was a possibility that the below normal values were caused by methoxyflurane. The patients remained clinically normal throughout.
There were 4 patients who had a systolic BP of less than 90 mm Hg after methoxyflurane inhalation. Three of these patients already had values at or below 90 mm Hg before methoxyflurane, and their BP only fell by 5 mm Hg after methoxyflurane. One patient had a decrease of 20 mm Hg in systolic BP after methoxyflurane. This patient had a systolic BP of 118 mm Hg before methoxyflurane that fell to 85 mm Hg within 10 minutes of receiving methoxyflurane, before rising to 95 mm Hg 20-30 minutes after methoxyflurane.
As with systolic BP, patients who exhibited low pulse rates after methoxyflurane inhalation also had low levels before inhalation. There were 27 patients who had subnormal levels of pulse rate after methoxyflurane inhalation. Twenty one of these patients already had values at, or below, 60 beats/min before methoxyflurane and their pulse rates only fell by 6 beats/min at the most after methoxyflurane. Six patients had falls of up to 10 beats/minute in pulse rate after methoxyflurane.
Their other vital signs, however, were within normal limits and they were not otherwise adversely affected by methoxyflurane inhalation or becoming symptomatically hypotensive.
No patients, either before or after methoxyflurane inhalation, exhibited respiratory rates of less than 10 breaths/minute.
Discussion
Overall, systolic BP fell slightly after methoxyflurane administration 0 to 10 minutes after administration
and then plateaued between 10 and 30 minutes after administration. All mean values for each time period were well above 90 mm Hg and within normal limits. Nearly all patients (>95%) both before and after methoxyflurane administration had levels of systolic BP within our normal limits. The proportion of patients that had values both above and below our defined normal levels decreased after methoxyflurane administration, and there was no evidence that methoxyflurane inhalation increased the probability of exhibiting abnormal systolic blood pressure.
In general, there was no particular pattern to the patients’ systolic BP levels after methoxyflurane administration across different age groups or indications. The few patients who exhibited low systolic BP after methoxyflurane inhalation tended also to have low levels before inhalation.
Overall, pulse rate fell continuously over 20 to 30 minutes after methoxyflurane administration. All mean values for each time period were well above 60 beats/minute and within normal limits. Most patients (>80%) both before and after methoxyflurane administration had pulse rates within normal limits. The proportion of patients that had values both above and below normal levels decreased after methoxyflurane administration, and the proportion of values within normal limits increased steadily between 0 and 30 minutes after methoxyflurane inhalation. There was no evidence that methoxyflurane inhalation increased the probability of exhibiting abnormal pulse rates.
The sharpest decrease was found in children under the age of 12; pulse rates in these patients fell by approximately 10 beats/min within 10 minutes of methoxyflurane administration, and remained around that level until at least 30
minutes after methoxyflurane administration. There was little overall pattern in pulse rate among patients presenting with different indications after methoxyflurane administration. As with systolic BP, patients who exhibited low pulse rates after methoxyflurane inhalation also had low levels before inhalation.
Overall, respiratory rate fell slightly over 20 to 30 minutes after methoxyflurane administration. All mean values for each time period were well above 10 breaths/minute and within normal limits. Most patients (>88%) both before and after methoxyflurane administration had respiratory rates within normal limits. The proportion of patients that
had raised levels decreased after methoxyflurane administration, and the proportion of values within normal limits increased steadily between 0 and 30 minutes after methoxyflurane inhalation. There was no evidence that methoxyflurane inhalation increased the probability of exhibiting abnormal respiratory rates.
In general, respiratory rate decreased slightly across age classes after methoxyflurane administration, except for a rise in adolescents. Consistent with the other vital signs measured, there was little overall pattern in respiratory rate among patients presenting with different indications. No patients, either before or after methoxyflurane inhalation,
exhibited respiratory rates of less than 10 breaths/ minute.
There were no cases of patients becoming symptomatic resulting from decreased systolic BP, pulse rate, or respiratory rate that was associated with methoxyflurane administration.
The evidence from recent clinical trials on methoxyflurane supports the results from this retrospective, observational study. No clinically significant changes were observed for vital signs (heart rate, respiratory rate, BP or temperature)
in the clinical study of methoxyflurane in patients undergoing a bone marrow biopsy procedure.6 Similarly, in the clinical study of methoxyflurane in patients presenting to an Emergency Department with minor trauma,7 there was little change in systolic BP, diastolic BP, respiratory rate, heart rate, heart rate rhythm (regular and irregular rhythm
specified) between the evaluations in patients in the methoxyflurane and placebo groups; indeed the results were comparable to that of the placebo group.
In a similar study to the present one, a retrospective, observational review of patient care record forms encompassing patients administered methoxyflurane or fentanyl for the prehospital management of presumed visceral pain was conducted by the Western Australian Ambulance Service between January 2004 and February 2006.8 They reported that methoxyflurane caused a mean reduction in systolic BP 5 minutes after the initial dose of methoxyflurane of 5.7 mm Hg (median 10 mm Hg). The effects of methoxyflurane on pulse and respiratory rates
were minimal, and consisted of slight falls in both parameters after methoxyflurane administration. In the majority of cases, methoxyflurane affected pulse rate favourably towards normal values, but the changes were not significant. Respiration was likewise affected minimally by methoxyflurane administration; mean respiration declined by 1.7
breaths/minute initially and by 2.1 breaths/minute on hospital arrival. No patients became bradypnoeic.5
One case (in forty) of hypotension was recorded in study of methoxyflurane use during emergency transportation. However this occurred following a surgical procedure, for which preoperative medication and an anaesthetic agent were administered.8
In a study in trauma patients, BP and heart rate remained stable following methoxyflurane administration, and in cases where the patient’s haemodynamic condition was initially impaired, there was an appreciable improvement i.e. increased differential BP and stronger heart beats. There was no respiratory depression, and breathing was
often normalised following the onset of analgesia. These observations led the authors to conclude that methoxyflurane has a stabilising effect on cardiorespiratory function and limits shock.7
In a long-term data linkage study, it was reported that in patients administered methoxyflurane no increased risk of disease occurrence was observed in any of the disease groups under investigation when compared with prehospital care patients who were not administered methoxyflurane.8 9
Conclusion
The administration of low dose methoxyflurane used for analgesia did not produce any deleterious effect on cardiovascular or respiratory parameters in this study whereby patients became symptomatic. The observations indicate that in this otherwise unselected, large group of presentations who had received methoxyflurane for pain, there were no deleterious effects on pulse rate, systolic BP, or respiratory rate, and this is supported by the
narrative notes. The initial changes in vital signs seen are consistent with reduction in pain and suffering. It is expected that elevated pulse rate, systolic BP or respiratory rate usually observed in patients with pain and suffering would decrease initially, and then plateau on commencement of analgesia as part of physiological responses to pain.
A precautionary statement in the Product Information document, warning against using methoxyflurane in patients with cardiovascular instability, or with respiratory impairment, appear largely unwarranted in the practical situation of pain relief. Such a warning may be an historical artefact of labelling from when methoxyflurane was used at much higher doses as an anaesthetic. There is no evidence, in this observational study of retrospective data, to indicate
that administration of methoxyflurane in low, controlled analgesic doses would result in decreased systolic blood pressure, pulse rate or respiratory rate of clinical significance. These findings are supported by the absence of any reports in recent clinical trials,1,2,6,7,8,9-16 in the literature, or with regulating authorities, that report such sensitivity, despite over four million doses dispensed during nearly forty years of use in Australia, and elsewhere.
The results of this study further support the conclusion that methoxyflurane is safe, simple to use and is an effective inhaled analgesic agent, with no significant physiological or toxic side effects at the prescribed analgesic doses.






